Abstract
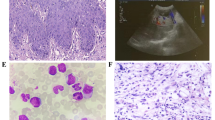
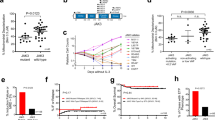
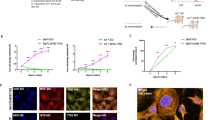
Aggressive NK-cell leukemia (ANKL) is a rare form of NK cell neoplasm that is more prevalent among people from Asia and Central and South America. Patients usually die within days to months, even after receiving prompt therapeutic management. Here we performed the first comprehensive study of ANKL by integrating whole genome, transcriptome and targeted sequencing, cytokine array as well as functional assays. Mutations in the JAK-STAT pathway were identified in 48% (14/29) of ANKL patients, while the extracellular STAT3 stimulator IL10 was elevated by an average of 56-fold (P < 0.0001) in the plasma of all patients examined. Additional frequently mutated genes included TP53 (34%), TET2 (28%), CREBBP (21%) and MLL2 (21%). Patient NK leukemia cells showed prominent activation of STAT3 phosphorylation, MYC expression and transcriptional activities in multiple metabolic pathways. Functionally, STAT3 activation and MYC expression were critical for the proliferation and survival of ANKL cells. STAT signaling regulated the MYC transcription program, and both STAT signaling and MYC transcription were required to maintain the activation of nucleotide synthesis and glycolysis. Collectively, the JAK-STAT pathway represents a major target for genomic alterations and IL10 stimulation in ANKL. This newly discovered JAK/STAT-MYC-biosynthesis axis may provide opportunities for the development of novel therapeutic strategies in treating this subtype of leukemia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chan J, Jaffe ES, Ralfkiaer E . Aggressive NK-cell leukaemia. In: Swerdlow S, Campo E, Harris N, eds. WHO Classification of Tumors of Heamatopoietic and Lymphoid Tissue. Lyon: International Agency for Research on Cancer, 2008:276–277.
Chan JK, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood 1997; 89:4501–4513.
Suzuki R, Suzumiya J, Nakamura S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia 2004; 18:763–770.
Suzuki R . Treatment of advanced extranodal NK/T cell lymphoma, nasal-type and aggressive NK-cell leukemia. Int J Hematol 2010; 92:697–701.
Nakashima Y, Tagawa H, Suzuki R, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer 2005; 44:247–255.
Ling S, Hu Z, Yang Z, et al. Extremely high genetic diversity in a single tumor points to prevalence of non-Darwinian cell evolution. Proc Natl Acad Sci USA 2015; 112:E6496–E6505.
Jiang L, Gu ZH, Yan ZX, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet 2015; 47:1061–1066.
Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013; 502:333–339.
Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 2011; 43:830–837.
Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368:2059–2074.
Leiserson MD, Vandin F, Wu HT, et al. Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nat Genet 2015; 47:106–114.
Dobashi A, Tsuyama N, Asaka R, et al. Frequent BCOR aberrations in extranodal NK/T-cell lymphoma, nasal type. Genes Chromosomes Cancer 2016; 55:460–471.
Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood 2012; 120:3048–3057.
Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012; 366:1905–1913.
Kucuk C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun 2015; 6:6025.
Bandapalli OR, Schuessele S, Kunz JB, et al. The activating STAT5B N642H mutation is a common abnormality in pediatric T-cell acute lymphoblastic leukemia and confers a higher risk of relapse. Haematologica 2014; 99:e188–e192.
Lopez C, Bergmann AK, Paul U, et al. Genes encoding members of the JAK-STAT pathway or epigenetic regulators are recurrently mutated in T-cell prolymphocytic leukaemia. Br J Haematol 2016; 173:265–273.
Pilati C, Amessou M, Bihl MP, et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med 2011; 208:1359–1366.
Gao LM, Zhao S, Liu WP, et al. Clinicopathologic characterization of aggressive natural killer cell leukemia involving different tissue sites. Am J Surg Pathol 2016; 40:836–846.
Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014; 371:1005–1015.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798–809.
Paull EO, Carlin DE, Niepel M, Sorger PK, Haussler D, Stuart JM . Discovering causal pathways linking genomic events to transcriptional states using Tied Diffusion through Interacting Events (TieDIE). Bioinformatics 2013; 29:2757–2764.
Ji H, Wu G, Zhan X, et al. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PLoS One 2011; 6:e26057.
Perna D, Faga G, Verrecchia A, et al. Genome-wide mapping of Myc binding and gene regulation in serum-stimulated fibroblasts. Oncogene 2012; 31:1695–1709.
Dang CV . MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med 2013; 3:a014217.
Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV . MYC, metabolism, and cancer. Cancer Discov 2015; 5:1024–1039.
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP . Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 2016; 14:11–31.
Loven J, Orlando DA, Sigova AA, et al. Revisiting global gene expression analysis. Cell 2012; 151:476–482.
Caro P, Kishan AU, Norberg E, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012; 22:547–560.
Li B, Qiu B, Lee DS, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 2014; 513:251–255.
Sellers K, Fox MP, Bousamra M, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 2015; 125:687–698.
Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci USA 2006; 103:18261–18266.
Yagita M, Huang CL, Umehara H, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 2000; 14:922–930.
Gong JH, Maki G, Klingemann HG . Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994; 8:652–658.
Schust J, Sperl B, Hollis A, Mayer TU, Berg T . Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 2006; 13:1235–1242.
Samanta M, Iwakiri D, Takada K . Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene 2008; 27:4150–4160.
Kitagawa N, Goto M, Kurozumi K, et al. Epstein-Barr virus-encoded poly(A)(-) RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J 2000; 19:6742–6750.
Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013; 122:3616–3627.
Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 2014; 123:1293–1296.
Vainchenker W, Plo I . TET2 loss, a rescue of JAK2V617F HSCs. Blood 2015; 125:212–213.
Chen E, Schneider RK, Breyfogle LJ, et al. Distinct effects of concomitant c expression and Tet2 loss in mice promote disease progression in myeloproliferative neoplasms. Blood 2015; 125:327–335.
Kameda T, Shide K, Yamaji T, et al. Loss of TET2 has dual roles in murine myeloproliferative neoplasms: disease sustainer and disease accelerator. Blood 2015; 125:304–315.
Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med 2015; 372:601–612.
Miklossy G, Hilliard TS, Turkson J . Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov 2013; 12:611–629.
Conacci-Sorrell M, McFerrin L, Eisenman RN . An overview of MYC and its interactome. Cold Spring Harb Perspect Med 2014; 4:a014357.
Kiuchi N, Nakajima K, Ichiba M, et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med 1999; 189:63–73.
Selvarajan V, Osato M, Nah GS, et al. RUNX3 is oncogenic in natural killer/T-cell lymphoma and is transcriptionally regulated by MYC. Leukemia 2017; 31:2219–2227.
Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013; 119:348–355.
Anders S, Huber W . Differential expression analysis for sequence count data. Genome Biol 2010; 11:R106.
Wang L, Feng Z, Wang X, Wang X, Zhang X . DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010; 26:136–138.
Huang DW, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102:15545–15550.
Wang Y, Song F, Zhu J, et al. GSA: Genome Sequence Archive. Genomics Proteomics Bioinformatics 2017; 15:14–18.
BIG Data Center Members. The BIG Data Center: from deposition to integration to translation. Nucleic Acids Res 2017; 45:D18–D24.
Acknowledgements
We thank all the faculties and staffs in the Clinical and Laboratory Unit of the Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their clinical and technical support; the Core Genomic Facility of Beijing Institute of Genomics, Chinese Academy of Sciences for the construction and sequencing of libraries; Drs Qing Li and Jiguang Wang for critical reading and valuable comments on the manuscript; Dr Kai Fu for providing the cell line YT. This study was supported by the National Natural Science Foundation of China (81570196 to JZ, 81425003 to QW, 81670152 to Liang H, 81600120 to NW, 81300410 to DW, 81500100 to YL and 81400122 to KZ), the National Key Basic Research Program of China (2014CB542001 to QW), the Key Program of the National Natural Science Foundation of China (81230052 to JZ), the Key Research Program of the Chinese Academy of Sciences (Precious Medicine Research in Chinese Population; KJZD-EW-L14-3 to QW), and the National High Technology Research and Development Program of China (863 program; 2012AA02A507 to JZ and 2014AA020532 to Liang H).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Strategies for the integrative analysis of ANKLs. (PDF 401 kb)
Supplementary information, Figure S2
Isolation of granulocytes, leukemia NK cells and normal NK cells. (PDF 387 kb)
Supplementary information, Figure S3
Frequency of somatic SNV of ANKL and 40 additional cancer types. (PDF 493 kb)
Supplementary information, Figure S4
EBV reads number of leukemia NK cells and granulocytes from 8 ANKL patients. (PDF 421 kb)
Supplementary information, Figure S5
KEGG pathway enrichment analysis of protein-altering somatic SNVs. (PDF 438 kb)
Supplementary information, Figure S6
Functional enrichment analysis of genomic alterations with HotNet2. (PDF 373 kb)
Supplementary information, Figure S7
STAT3 and STAT5B mutations identified in ANKL patients. (PDF 541 kb)
Supplementary information, Figure S8
TET2 mutations identified in ANKL patients. (PDF 471 kb)
Supplementary information, Figure S9
Semi-quantitative immunoreactivity histological scores of MYC staining in bone marrow biopsy of ANKL and control samples. (PDF 422 kb)
Supplementary information, Figure S10
Functional enrichment map for MYC-signature genes that were upregulated in ANKLs compared to healthy donors. (PDF 856 kb)
Supplementary information, Figure S11
The effect of IL10 or a STAT3 inhibitor (Stattic) on the apoptosis of ANKL cell lines. (PDF 457 kb)
Supplementary information, Figure S12
The effect of IL10 and the STAT3 inhibitor (Stattic) on the mRNA expression of MYC in ANKL cell lines. (PDF 470 kb)
Supplementary information, Figure S13
The rate of EdU incorporation in JQ1-treated ANKL cell lines. (PDF 404 kb)
Supplementary information, Figure S14
Gene-set enrichment analysis (GSEA) of known MYC and STAT3 signatures. (PDF 523 kb)
Supplementary information, Figure S15
Enrichment of metabolic pathways in primary ANKL leukemia cells and ANKL cell lines. (PDF 480 kb)
Supplementary information, Figure S16
The effect of STAT3 Y640F mutant on the phosphorylation of STAT3 and mRNA expression of MYC target gene. (PDF 583 kb)
Supplementary information, Figure S17
The effect of IL10 on the mRNA expression of MYC and MYC target genes in KHYG-1 cell line transfected with STAT3 Y640F mutant. (PDF 584 kb)
Supplementary information, Figure S18
The effect of IL10 on STAT3 phosphorylation, MYC expressionand the proliferation of STAT3 Y640F-mutant ANKL cell line YT. (PDF 505 kb)
Supplementary information, Figure S19
Quantitative analysis of the Epstein-Barr virus (EBV) load in ANKL patients and healthy donors with whole-transcriptome sequencing (WTS) data. (PDF 351 kb)
Supplementary information, Figure S20
Expression of EBV-encoded small RNAs in primary ANKL leukemia cells. (PDF 400 kb)
Supplementary information, Figure S21
A large gain across MYC and an inter-chromosomal translocation detected by GVC-CNV and GVC-SV in ANKL No.19. (PDF 456 kb)
Supplementary information, Table S1
Patient characteristics of ANKL. (PDF 633 kb)
Supplementary information, Table S2
SNVs identified in ANKL NK leukemia cells. (PDF 412 kb)
Supplementary information, Table S3
CNVs identified in ANKL NK leukemia cells. (PDF 6359 kb)
Supplementary information, Table S4
SVs identified in ANKL NK leukemia cells. (PDF 3596 kb)
Supplementary information, Table S5
AmpliSeq targeted sequencing results of ANKLs. (PDF 630 kb)
Supplementary information, Table S7
H-score of phosphorylated STAT3 and corresponding STAT mutation status of ANKL cases. (PDF 369 kb)
Supplementary information, Table S8
Differentially upregulated genes in ANKL leukemia cells in comparison with normal controls. (PDF 730 kb)
Supplementary information, Table S9
Differentially downregulated genes in ANKL leukemia cells in comparison with normal controls. (PDF 1385 kb)
Supplementary information, Table S10
KEGG pathway enrichment analysis of upregulated genes in ANKL leukemia cells. (PDF 548 kb)
Supplementary information, Table S11
KEGG pathway enrichment analysis of downregulated genes in ANKL leukemia cells. (PDF 398 kb)
Supplementary information, Table S12
IPA metabolic pathways analysis of ANKL and tumors that have known metabolic features. (PDF 1531 kb)
Supplementary information, Table S13
Sequencing depth information of 8 WGS ANKL patients. (PDF 430 kb)
Supplementary information, Table S14
Primers for quantitative RT-PCR (PDF 319 kb)
Supplementary information, Data S1
Materials and methods (PDF 1056 kb)
Rights and permissions
About this article
Cite this article
Huang, L., Liu, D., Wang, N. et al. Integrated genomic analysis identifies deregulated JAK/STAT-MYC-biosynthesis axis in aggressive NK-cell leukemia. Cell Res 28, 172–186 (2018). https://doi.org/10.1038/cr.2017.146
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.146
Keywords
This article is cited by
-
Genetic and clinical distinction between aggressive NK-cell leukemia and extranodal NK/T-cell lymphoma with bone marrow involvement
Scientific Reports (2025)
-
Biphenotypic NK-Large granular lymphocytic leukemia with aggressive clinical features: a case report and literature review
Annals of Hematology (2025)
-
Targeted inhibition of DHODH is synergistic with BCL2 blockade in HGBCL with concurrent MYC and BCL2 rearrangement
BMC Cancer (2024)
-
Initial diagnosis of extranodal NK/T-cell lymphoma in pericardial fluid with concomitant hemophagocytic lymphohistiocytosis (HLH)
Journal of Hematopathology (2024)
-
Amino acid influx via LAT1 regulates iron demand and sensitivity to PPMX-T003 of aggressive natural killer cell leukemia
Leukemia (2024)