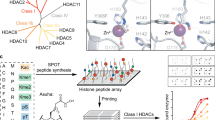
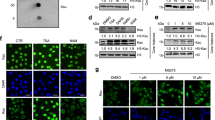
Abstract
Recent studies on enzymes and reader proteins for histone crotonylation support a function of histone crotonylation in transcription. However, the enzyme(s) responsible for histone decrotonylation (HDCR) remains poorly defined. Moreover, it remains to be determined if histone crotonylation is physiologically significant and functionally distinct from or redundant to histone acetylation. Here we present evidence that class I histone deacetylases (HDACs) rather than sirtuin family deacetylases (SIRTs) are the major histone decrotonylases, and that histone crotonylation is as dynamic as histone acetylation in mammalian cells. Notably, we have generated novel HDAC1 and HDAC3 mutants with impaired HDAC but intact HDCR activity. Using these mutants we demonstrate that selective HDCR in mammalian cells correlates with a broad transcriptional repression and diminished promoter association of crotonylation but not acetylation reader proteins. Furthermore, we show that histone crotonylation is enriched in and required for self-renewal of mouse embryonic stem cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Roth SY, Denu JM, Allis CD . Histone acetyltransferases. Annu Rev Biochem 2001; 70:81–120.
Wolffe AP . Histone deacetylase: a regulator of transcription. Science 1996; 272:371–372.
Rousseaux S, Khochbin S . Histone acylation beyond acetylation: terra incognita in chromatin biology. Cell J 2015; 17:1–6.
Tan M, Luo H, Lee S, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011; 146:1016–1028.
Olsen CA . Expansion of the lysine acylation landscape. Angew Chem Int Ed Engl 2012; 51:3755–3756.
Dai L, Peng C, Montellier E, et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat Chem Biol 2014; 10:365–370.
Zhang K, Chen Y, Zhang Z, Zhao Y . Identification and verification of lysine propionylation and butyrylation in yeast core histones using PTMap software. J Proteome Res 2009; 8:900–906.
Xie Z, Dai J, Dai L, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics 2012; 11:100–107.
Sabari BR, Zhang D, Allis CD, Zhao Y . Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol 2017; 18:90–101.
Sabari BR, Tang Z, Huang H, et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell 2015; 58:203–215.
Li Y, Sabari BR, Panchenko T, et al. Molecular coupling of histone crotonylation and active transcription by AF9 YEATS domain. Mol Cell 2016; 62:181–193.
Xiong X, Panchenko T, Yang S, et al. Selective recognition of histone crotonylation by double PHD fingers of MOZ and DPF2. Nat Chem Biol 2016; 12:1111–1118.
Andrews FH, Shinsky SA, Shanle EK, et al. The Taf14 YEATS domain is a reader of histone crotonylation. Nat Chem Biol 2016; 12:396–398.
Li Y, Wen H, Xi Y, et al. AF9 YEATS domain links histone acetylation to DOT1L-mediated H3K79 methylation. Cell 2014; 159:558–571.
Zeng L, Zhang Q, Li S, Plotnikov AN, Walsh MJ, Zhou MM . Mechanism and regulation of acetylated histone binding by the tandem PHD finger of DPF3b. Nature 2010; 466:258–262.
Zhao D, Guan H, Zhao S, et al. YEATS2 is a selective histone crotonylation reader. Cell Res 2016; 26:629–632.
Cheng Z, Tang Y, Chen Y, et al. Molecular characterization of propionyllysines in non-histone proteins. Mol Cell Proteomics 2009; 8:45–52.
Smith BC, Denu JM . Acetyl-lysine analog peptides as mechanistic probes of protein deacetylases. J Biol Chem 2007; 282:37256–37265.
Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011; 334:806–809.
Park J, Chen Y, Tishkoff DX, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 2013; 50:919–930.
Rardin MJ, He W, Nishida Y, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 2013; 18:920–933.
Tan M, Peng C, Anderson KA, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 2014; 19:605–617.
Feldman JL, Baeza J, Denu JM . Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem 2013; 288:31350–31356.
Bao X, Wang Y, Li X, et al. Identification of 'erasers' for lysine crotonylated histone marks using a chemical proteomics approach. eLife 2014; 3. doi: 10.7554/eLife.02999
Madsen AS, Olsen CA . Profiling of substrates for zinc-dependent lysine deacylase enzymes: HDAC3 exhibits decrotonylase activity in vitro. Angew Chem Int Ed Engl 2012; 51:9083–9087.
Van der Veer E, Ho C, O'Neil C, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem 2007; 282:10841–10845.
Lin CY, Loven J, Rahl PB, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012; 151:56–67.
Loven J, Orlando DA, Sigova AA, et al. Revisiting global gene expression analysis. Cell 2012; 151:476–482.
Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K . The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci USA 2003; 100:8758–8763.
Filippakopoulos P, Picaud S, Mangos M, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 2012; 149:214–231.
Kimura H, Tada M, Nakatsuji N, Tada T . Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol 2004; 24:5710–5720.
Moussaieff A, Rouleau M, Kitsberg D, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 2015; 21:392–402.
Niwa H, Miyazaki J, Smith AG . Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24:372–376.
Young RA . Control of the embryonic stem cell state. Cell 2011; 144:940–954.
Fang L, Zhang L, Wei W, et al. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol Cell 2014; 55:537–551.
Li Y, Zhao D, Chen Z, Li H . YEATS domain: linking histone crotonylation to gene regulation. Transcription 2017; 8:9–14.
Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D . Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 1997; 89:357–364.
Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE . Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 1997; 89:341–347.
Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN . Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 1997; 89:349–356.
Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W . NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell 1998; 2:851–861.
Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D . The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 1998; 95:279–289.
Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R . A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 2000; 14:1048–1057.
Li J, Wang J, Wang J, et al. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 2000; 19:4342–4350.
Kaczmarska Z, Ortega E, Goudarzi A, et al. Structure of p300 in complex with acyl-CoA variants. Nat Chem Biol 2017; 13:21–29.
Yao TP, Oh SP, Fuchs M, et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 1998; 93:361–372.
Chelmicki T, Dundar F, Turley MJ, et al. MOF-associated complexes ensure stem cell identity and Xist repression. eLife 2014; 3:e02024.
Li X, Li L, Pandey R, et al. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell 2012; 11:163–178.
Shechter D, Dormann HL, Allis CD, Hake SB . Extraction, purification and analysis of histones. Nat Protoc 2007; 2:1445–1457.
Liu X, Gao Q, Li P, et al. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat Commun 2013; 4:1563.
Yoon HG, Choi Y, Cole PA, Wong J . Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol Cell Biol 2005; 25:324–335.
Millard CJ, Watson PJ, Celardo I, et al. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell 2013; 51:57–67.
Schuetz A, Min J, Allali-Hassani A, et al. Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity. J Biol Chem 2008; 283:11355–11363.
Vannini A, Volpari C, Gallinari P, et al. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8-substrate complex. EMBO Rep 2007; 8:879–884.
Liu XG, Wei W, Liu YT, et al. MOF as an evolutionarily conserved histone crotonyltransferase and transcriptional activation by histone acetyltransferase-deficient and crotonyltransferase-competent CBP/p300. Cell Discov 2017; 3:17016. doi:10.1038/celldisc.2017.16
Acknowledgements
We thank members of Wong's laboratory for valuable discussion. We thank Dr Cheng-Ming Chiang (UT Southwestern) for critical reading of the manuscript. This study is supported by grants from the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” of China (2014ZX09507002-005 to JW), the Ministry of Science and Technology of China (2015CB910402 to JW), the National Natural Science Foundation of China (81530078 and 31571325 to JW), the Science and Technology Commission of Shanghai Municipality (14XD1401700 and 11DZ2260300 to JW).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Characterization of pan-Kcr antibodies. (PDF 631 kb)
Supplementary information, Figure S2
The class II HDACs and multiple SIRTs are inactive in histone decrotonylation. (PDF 1458 kb)
Supplementary information, Figure S3
The SIRT2 and SIRT3 are inactive in histone decrotonylation. (PDF 1169 kb)
Supplementary information, Figure S4
Knockdown of SIRT1, SIRT3 or SIRT1/3/5 in combination has no obvious effect on global histone acetylation and crotonylation. (PDF 534 kb)
Supplementary information, Figure S5
TSA treatment results in a similar kinetic increase of histone acetylation and crotonylation in mammalian cells. (PDF 598 kb)
Supplementary information, Figure S6
The same enzymatic center catalyzes histone deacetylation and decrotonylation for SIRT1 and HDCA1. (PDF 819 kb)
Supplementary information, Figure S7
Various HDAC1 mutations result in loss of both HDAC and HDCR activities. (PDF 1253 kb)
Supplementary information, Figure S8
The HDAC1-VRPP mutantis active in decrotonylation of multiple sites of his tone crotonylation. (PDF 611 kb)
Supplementary information, Figure S9
The HDAC1-VRPP mutant is active in transcriptional repression. (PDF 506 kb)
Supplementary Table S1
Sequences for qRT-PCR primers (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Wei, W., Liu, X., Chen, J. et al. Class I histone deacetylases are major histone decrotonylases: evidence for critical and broad function of histone crotonylation in transcription. Cell Res 27, 898–915 (2017). https://doi.org/10.1038/cr.2017.68
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.68
Keywords
This article is cited by
-
Lysine crotonylation in disease: mechanisms, biological functions and therapeutic targets
Epigenetics & Chromatin (2025)
-
VEGFR3 mitigates hypertensive nephropathy by enhancing mitophagy via regulating crotonylation of HSPA1L
Cell Communication and Signaling (2025)
-
Mechanistic insights into post-translational modifications in hepatic fibrosis: pathogenic roles and therapeutic potentials
Journal of Translational Medicine (2025)
-
Novel histone modifications and liver cancer: emerging frontiers in epigenetic regulation
Clinical Epigenetics (2025)
-
Epigenetic regulators in cancer therapy and progression
npj Precision Oncology (2025)