Abstract
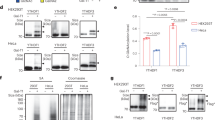
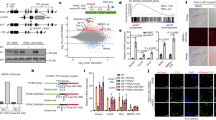
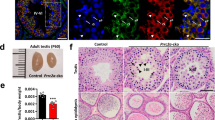
N6-methyladenosine (m6A) is the most common internal modification in eukaryotic mRNA. It is dynamically installed and removed, and acts as a new layer of mRNA metabolism, regulating biological processes including stem cell pluripotency, cell differentiation, and energy homeostasis. m6A is recognized by selective binding proteins; YTHDF1 and YTHDF3 work in concert to affect the translation of m6A-containing mRNAs, YTHDF2 expedites mRNA decay, and YTHDC1 affects the nuclear processing of its targets. The biological function of YTHDC2, the final member of the YTH protein family, remains unknown. We report that YTHDC2 selectively binds m6A at its consensus motif. YTHDC2 enhances the translation efficiency of its targets and also decreases their mRNA abundance. Ythdc2 knockout mice are infertile; males have significantly smaller testes and females have significantly smaller ovaries compared to those of littermates. The germ cells of Ythdc2 knockout mice do not develop past the zygotene stage and accordingly, Ythdc2 is upregulated in the testes as meiosis begins. Thus, YTHDC2 is an m6A-binding protein that plays critical roles during spermatogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Moore MJ . From birth to death: the complex lives of eukaryotic mRNAs. Science 2005; 309:1514–1518.
Desrosiers R, Friderici K, Rottman F . Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA 1974; 71:3971–3975.
Zhao BS, Roundtree IA, He C . Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 2016; 18:31–42.
Wei C, Moss B . Nucleotide Sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 1977; 16:1672–1676.
Wei C, Gershowitz A, Moss B . 5′-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry 1976; 15:397–401.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485:201–206.
Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 2014; 10:93–95.
Wang X, Feng J, Xue Y, et al. Structural basis of N6-adenosine methylation by the METTL3-METTL14 complex. Nature 2016; 534:1–15.
Wang P, Doxtader KA, Nam Y . Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Molecular Cell 2016; 63:306–317
Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011; 7:885–887.
Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 2013; 49:18–29.
Ping X, Sun B, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 2014; 24:177–189.
Geula S, Moshitch-Moshkovitz S, Dominissini D, et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015; 347:1002–1006.
Fustin JM, Doi M, Yamaguchi Y, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 2013; 155:793–806.
Batista PJ, Molinie B, Wang J, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 2014; 15:707–719.
Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009; 458:894–898.
Li Z, Weng H, Su R, et al. FTO Plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine rna demethylase. Cancer Cell 2017; 31:127–141.
Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014; 505:117–120.
Wang X, Zhao BS, Roundtree IA, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015; 161:1388–1399.
Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res 2017; 27:315–328.
Xu C, Wang X, Liu K, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 2014; 10:927–929.
Xiao W, Adhikari S, Dahal U, et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell 2016; 61:507–519.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T . N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015; 518:560–564.
Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB . A mRNA methylation directs translational control of heat shock response. Nature 2015; 526:591–594.
Zhao BS, Wang X, Beadell A V, et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 2017; 542:475–478.
Wang X, Zhao BS, Roundtree IA, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015; 161:1388–1399.
Li A, Chen YS, Ping XL, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res 2017; 27:444–447.
Du H, Zhao Y, He J, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 2016; 7:12626.
Morohashi K, Sahara H, Watashi K, et al. Cyclosporin A associated helicase-like protein facilitates the association of hepatitis C virus RNA polymerase with its cellular cyclophilin B. PLoS One 2011; 6:e18285.
Abby E, Tourpin S, Ribeiro J, et al. Implementation of meiosis prophase I programme requires a conserved retinoid-independent stabilizer of meiotic transcripts. Nat Commun 2016; 7:10324.
Soh YQS, Mikedis MM, Kojima M, Godfrey AK, Rooij G De, Page DC . Meioc maintains an extended meiotic prophase I in mice. PLoS Genet 2017; 13:e1006704.
Kerr JB, Loveland KL, O'Bryan MK, De Kretzker DM . Cytology of the testis and intrinsic control mechanisms. In: Knobil and Neill's Physiology of Reproduction. 2006, 25:827–947.
Zhu T, Roundtree IA, Wang P, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res 2014; 24:1493–1496.
Wang X, He C . Dynamic RNA modifications in posttranscriptional regulation. Mol Cell 2014; 56:5–12.
Popp MWL, Maquat LE . Organizing principles of mammalian nonsense-mediated mRNA decay. Annu Rev Genet 2013; 47:139–165.
Brogna S, Wen J . Nonsense-mediated mRNA decay (NMD) mechanisms. Nat Struct Mol Biol 2009; 16:107–113.
Gregersen LH, Schueler M, Munschauer M, et al. MOV10 Is a 5′ to 3′ RNA Helicase Contributing to UPF1 mRNA target degradation by translocation along 3′ UTRs. Mol Cell 2014; 54:573–585.
Nagarajan VK, Jones CI, Newbury SF, Green PJ . XRN 5′→3′ exoribonucleases: Structure, mechanisms and functions. Biochim Biophys Acta - Gene Regul Mech 2013; 1829:590–603.
Li F, Zhao D, Wu J, Shi Y . Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Res 2014; 24:1490–1492.
Roundtree IA, Evans ME, Pan T, Chuan He . Dynamic RNA modifications in gene expression regulation. Cell 2017; 169:1187.
Monesi V . Ribonucleic acid synthesis during mitosis and meiosis in the mouse testis. J Cell Biol 1964; 22:521–532.
Page J, Fuente R de la, Manterola M, et al. Inactivation or non-reactivation: what accounts better for the silence of sex chromsoomes during mammalian male meiosis? Chromosoma 2012; 121:307–326.
Fanale D, Iovanna JL, Calvo EL, et al. Germline copy number variation in the YTHDC2 gene: does it have a role in finding a novel potential molecular target involved in pancreatic adenocarcinoma susceptibility? Expert Opin Ther Targets 2014; 18:841–850.
Tanabe A, Tanikawa K, Tsunetomi M, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett 2016; 376:34–42.
Shen B, Zhang W, Zhang J, et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods 2014; 11:399–402.
Hafner M, Landthaler M, Burger L, et al. PAR-CliP — A method to identify transcriptome-wide the binding sites of rna binding proteins. J Vis Exp 2010 Jul 2. pii:2034. doi: 10.3791/2034
Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M . A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods 2011; 8:559–564.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL . TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 2013; 14:R36.
Trapnell C, Roberts A, Goff L, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 2012; 7:562–578.
Robinson MD, McCarthy DJ, Smyth GK . edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009; 26:139–140.
Corcoran DL, Georgiev S, Mukherjee N, et al. PARalyzer: definition of RNA binding sites from PAR-CLIP short-read sequence data. Genome Biol 2011; 12:R79.
Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and b cell identities. Mol Cell 2010; 38:576–589.
Bazzini AA, Lee MT, Giraldez AJ . Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 2012; 336:233–237.
Shi Y, Sawada J, Sui G, et al. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 2003; 422:735–738.
Acknowledgements
We thank Prof Tao Pan (The University of Chicago) for kindly providing the polysome profiling instrument. We thank Peter Chen and Phil Xie for technical assistance. We also thank the Institutes of Biomedical Sciences at Fudan University for the protein mass spectrometry experiments. This work was supported by the National Science Fund for Excellent Young Scholars (31622039 to BS), the National Key Research and Development Program of China (2016YFC1000600 to BS, 2016YFA0500902 to ML, 2017YFA0103803 to JS), the Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20160045 to BS), and National Institutes of Health grants HG008935 and GM113194 (to CH). CH is an investigator of the Howard Hughes Medical Institute (HHMI). HM is supported by the Postdoctoral International Exchange Program of the China Postdoctoral Council (CPC). PH is supported by the University of Chicago Medical Scientist Training Program (MSTP; NIH MSTP training grant T32GM007281).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Domains of YTHDC2 (https://www.ncbi.nlm.nih.gov/gene/64848). (PDF 39 kb)
Supplementary information, Figure S2
(Supplement to Figure 1) YTHDC2 binds preferentially to m6A-marked RNA transcripts. (PDF 148 kb)
Supplementary information, Figure S3
Characterization of Ythdc2−/− mouse. (PDF 105 kb)
Supplementary information, Figure S4
Female Ythdc2−/− mice demonstrate defects in the ovaries. (PDF 152 kb)
Supplementary information, Figure S5
Expression of Ythdc2 protein in wild type testes over time. (PDF 110 kb)
Supplementary information, Figure S6
Meiotic defects in Ythdc2-deficient meiocytes. (PDF 195 kb)
Supplementary information, Figure S7
Comparative histological analysis of testes of young Ythdc2+/+ and Ythdc2−/− mice. (PDF 436 kb)
Supplementary information, Figure S8
Ythdc2-deficient mice show normal spermatogonia. (PDF 199 kb)
Supplementary information, Figure S9
Ythdc2-deficient mice show normal sertoli cells. (PDF 445 kb)
Supplementary information, Figure S10
(Supplement to Figure 4) YTHDC2 affects the translation efficiency and stability of its targets. (PDF 141 kb)
Supplementary information, Figure S11
m6A-seq of 8.5 d.p.p. Ythdc2+/+ and Ythdc2−/− testes. (PDF 107 kb)
Supplementary information, Figure S12
Effect of YTHDC2 on mRNA abundance and translation efficiency may be m6A dependent. (PDF 108 kb)
Supplementary information, Figure S13
Confirmation of components of protein complex containing YTHDC2 obtained from tandem affinity purification followed by western blot. (PDF 111 kb)
Supplementary information, Table S1
Target transcripts of Ythdc2 in adult mouse testes, determined by CLIP-seq (XLSX 1895 kb)
Supplementary information, Table S2
Target transcripts of Ythdc2 in 16 d.p.p. mouse testes, determined by RIP-seq (XLSX 1406 kb)
Supplementary information, Table S3
Top 40 GO terms of Ythdc2 RIP-seq targets in mouse testes (XLSX 48 kb)
Supplementary information, Table S4
m6A-seq of control and Ythdc2−/− mice (XLSX 8512 kb)
Supplementary information, Table S5
Targets of YTHDC2 in HeLa cells, determined by PAR-CLIP (XLSX 120 kb)
Supplementary information, Table S6
Binding partner proteins of YTHDC2 in HeLa cells, determined by Tandem Affinity Purification followed by mass spectrometry (XLSX 1107 kb)
Rights and permissions
About this article
Cite this article
Hsu, P., Zhu, Y., Ma, H. et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27, 1115–1127 (2017). https://doi.org/10.1038/cr.2017.99
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cr.2017.99
Keywords
This article is cited by
-
Molecular mechanisms of m6A modifications regulating tumor radioresistance
Molecular Medicine (2025)
-
Optimization of in vivo delivery methods and their applications in seminiferous tubules of mice
BMC Biotechnology (2025)
-
Baicalin targets YTHDC2 and alleviates male reproductive toxicity caused by co-exposure to nanoplastics and manganese through m6A-dependent pathway
Journal of Nanobiotechnology (2025)
-
Epitranscriptomic regulation of HIF-1: bidirectional regulatory pathways
Molecular Medicine (2025)
-
Exploring diagnostic m6A regulators in primary open-angle glaucoma: insight from gene signature and possible mechanisms by which key genes function
BMC Medical Genomics (2025)