Abstract
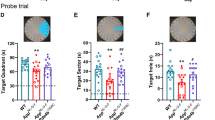
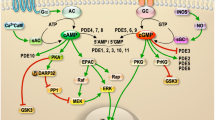
Bilateral adrenocortical hyperplasia (BAH) is the second most common cause of corticotropin-independent Cushing syndrome (CS). Genetic forms of BAH have been associated with complex syndromes such as Carney Complex and McCune–Albright syndrome or may present as isolated micronodular adrenocortical disease (iMAD) usually in children and young adults with CS. A genome-wide association study identified inactivating phosphodiesterase (PDE) 11A (PDE11A)-sequencing defects as low-penetrance predisposing factors for iMAD and related abnormalities; we also described a mutation (c.914A>C/H305P) in cyclic AMP (cAMP)-specific PDE8B, in a patient with iMAD. In this study we further characterize this mutation; we also found a novel PDE8B isoform that is highly expressed in the adrenal gland. This mutation is shown to significantly affect the ability of the protein to degrade cAMP in vitro. Tumor tissues from patients with iMAD and no mutations in the coding PDE8B sequence or any other related genes (PRKAR1A, PDE11A) showed downregulated PDE8B expression (compared to normal adrenal cortex). Pde8b is detectable in the adrenal gland of newborn mice and is widely expressed in other mouse tissues. We conclude that PDE8B is another PDE gene linked to iMAD; it is a candidate causative gene for other adrenocortical lesions linked to the cAMP signaling pathway and possibly for tumors in other tissues.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stratakis CA, Boikos SA : Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab 2007; 3: 748–757.
Gunther DF, Bourdeau I, Matyakhina L et al: Cyclical Cushing syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab 2004; 89: 3173–3182.
Stratakis CA : Adrenocortical tumors, primary pigmented adrenocortical disease (PPNAD)/Carney complex, and other bilateral hyperplasias: the NIH studies. Horm Metab Res 2007; 39: 467–473.
Weinstein LS, Yu S, Warner DR, Liu J : Endocrine manifestations o stimulatory G protein-subunit mutations and the role of genomic imprinting. Endocr Rev 2001; 22: 675–705.
Kirschner LS, Carney JA, Pack SD et al: Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 2000; 26: 89–92.
Bourdeau I, Stratakis CA : Cyclic AMP-dependent signaling aberrations in macronodular adrenal disease. Ann N Y Acad Sci 2002; 968: 240–255.
Horvath A, Boikos S, Giatzakis C et al: A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 2006; 38: 794–800.
Fawcett L, Baxendale R, Stacey P et al: Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA 2000; 97: 3702–3707.
Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K : Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem 2000; 275: 31469–31479.
Hetman JM, Robas N, Baxendale R et al: Cloning and characterization of two splice variants of human phosphodiesterase 11A. Proc Natl Acad Sci USA 2000; 97: 12891–12895.
Loughney K, Taylor J, Florio VA : 3′,5′-cyclic nucleotide phosphodiesterase 11A: localization in human tissues. Int J Impot Res 2005; 17: 320–325.
Diaz A, Danon M, Crawford J : McCune–Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab 2007; 20: 853–880.
Horvath A, Mericq V, Stratakis CA : Mutation in PDE8B, a cAMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med 2008; 358: 750–752.
Horvath A, Giatzakis C, Robinson-White A et al: Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res 2006; 66: 11571–11575.
Soderling SH, Bayuga SJ, Beavo JA : Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA 1998; 95: 8991–8996.
Hayashi M, Shimada Y, Nishimura Y, Hama T, Tanaka T : Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun 2002; 297: 1253–1258.
Gamanuma M, Yuasa K, Sasaki T, Sakurai N, Kotera J, Omori K : Comparison of enzymatic characterization and gene organization of cyclic nucleotide phosphodiesterase 8 family in humans. Cell Signal 2003; 15: 565–574.
Conti M, Beavo J : Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 2007; 76: 481–511.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM : Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432.
Kim S, Moustaid-Moussa N : Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr 2000; 130: 3110S–3115S.
Trayhurn P, Beattie JH : Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60: 329–339.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A et al: Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 2003; 100: 14211–14216.
Lamounier-Zepter V, Bornstein SR, Kunes J et al: Adrenocortical changes and arterial hypertension in lipoatrophic A-ZIP/F-1 mice. Mol Cell Endocrinol 2008; 280: 39–46.
Haluzik M, Dietz KR, Kim JK et al: Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes 2002; 51: 2113–2118.
Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC : Leptin interferes with adrenocorticotropin/3′,5′-cyclic adenosine monophosphate (cAMP) signaling, possibly through a Janus kinase 2-phosphatidylinositol 3-kinase/Akt-phosphodiesterase 3-cAMP pathway, to down-regulate cholesterol side-chain cleavage cytochrome P450 enzyme in human adrenocortical NCI-H295 cell line. J Clin Endocrinol Metab 2006; 91: 2761–2769.
Acknowledgements
It was supported by US NIH intramural project Z01-HD-000642-04 to CAS and, in part, by Groupement d'Intérêt Scientifique-Institut National de la Santé et de la Recherche Médicale Institut des Maladies Rares and the Plan Hospitalier de Recherche Clinique (AOM 02068) to the Comete Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare that they have no competing financial interests.
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Horvath, A., Giatzakis, C., Tsang, K. et al. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet 16, 1245–1253 (2008). https://doi.org/10.1038/ejhg.2008.85
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2008.85
Keywords
This article is cited by
-
Retinoblastoma gene expression profiling based on bioinformatics analysis
BMC Medical Genomics (2023)
-
Overview of the 2022 WHO Classification of Adrenal Cortical Tumors
Endocrine Pathology (2022)
-
What Did We Learn from the Molecular Biology of Adrenal Cortical Neoplasia? From Histopathology to Translational Genomics
Endocrine Pathology (2021)
-
A genetic and molecular update on adrenocortical causes of Cushing syndrome
Nature Reviews Endocrinology (2016)
-
Regulation of the adrenocortical stem cell niche: implications for disease
Nature Reviews Endocrinology (2015)