Abstract
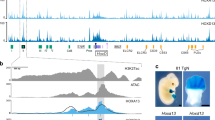
Léri-Weill Dyschondrosteosis (LWD) is a dominant skeletal disorder characterized by short stature and distinct bone anomalies. SHOX gene mutations and deletions of regulatory elements downstream of SHOX resulting in haploinsufficiency have been found in patients with LWD. SHOX encodes a homeodomain transcription factor and is known to be expressed in the developing limb. We have now analyzed the regulatory significance of the region upstream of the SHOX gene. By comparative genomic analyses, we identified several conserved non-coding elements, which subsequently were tested in an in ovo enhancer assay in both chicken limb bud and cornea, where SHOX is also expressed. In this assay, we found three enhancers to be active in the developing chicken limb, but none were functional in the developing cornea. A screening of 60 LWD patients with an intact SHOX coding and downstream region did not yield any deletion of the upstream enhancer region. Thus, we speculate that SHOX upstream deletions occur at a lower frequency because of the structural organization of this genomic region and/or that SHOX upstream deletions may cause a phenotype that differs from the one observed in LWD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Belin V, Cusin V, Viot G et al: SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet 1998; 19: 67–69.
Shears DJ, Vassal HJ, Goodman FR et al: Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet 1998; 19: 70–73.
Rao E, Weiss B, Fukami M et al: Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 1997; 16: 54–63.
Schiller S, Spranger S, Schechinger B et al: Phenotypic variation and genetic heterogeneity in Leri-Weill syndrome. Eur J Hum Genet 2000; 8: 54–62.
Cormier-Daire V, Huber C, Munnich A : Allelic and nonallelic heterogeneity in dyschondrosteosis (Leri-Weill syndrome). Am J Med Genet 2001; 106: 272–274.
Clement-Jones M, Schiller S, Rao E et al: The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum Mol Genet 2000; 9: 695–702.
Huber C, Rosilio M, Munnich A, Cormier-Daire V : High incidence of SHOX anomalies in individuals with short stature. J Med Genet 2006; 43: 735–739.
Rappold G, Blum WF, Shavrikova EP et al: Genotypes and phenotypes in children with short stature: clinical indicators of SHOX haploinsufficiency. J Med Genet 2007; 44: 306–313.
Chen J, Wildhardt G, Zhong Z et al: Enhancer mutations of the SHOX gene as a frequent cause of short stature – the essential role of a 250 kb downstream regulatory domain. J Med Genet 2009; 46: 834–839.
Sabherwal N, Bangs F, Roth R et al: Long-range conserved non-coding SHOX sequences regulate expression in developing chicken limb and are associated with short stature phenotypes in human patients. Hum Mol Genet 2007; 16: 210–222.
Benito-Sanz S, Thomas NS, Huber C et al: A novel class of Pseudoautosomal region 1 deletions downstream of SHOX is associated with Leri-Weill dyschondrosteosis. Am J Hum Genet 2005; 77: 533–544.
Fukami M, Kato F, Tajima T, Yokoya S, Ogata T : Transactivation function of an approximately 800-bp evolutionarily conserved sequence at the SHOX 3′ region: implication for the downstream enhancer. Am J Hum Genet 2006; 78: 167–170.
Timmer J, Johnson J, Niswander L : The use of in ovo electroporation for the rapid analysis of neural-specific murine enhancers. Genesis 2001; 29: 123–132.
Belo JA, Bouwmeester T, Leyns L et al: Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev 1997; 68: 45–57.
Tiecke E, Bangs F, Blaschke R, Farrell ER, Rappold G, Tickle C : Expression of the short stature homeobox gene Shox is restricted by proximal and distal signals in chick limb buds and affects the length of skeletal elements. Dev Biol 2006; 298: 585–596.
Bleyl SB, Byrne JL, South ST et al: Brachymesomelic dysplasia with Peters anomaly of the eye results from disruptions of the X chromosome near the SHOX and SOX3 genes. Am J Med Genet A 2007; 143A: 2785–2795.
Kleinjan DA, van Heyningen V : Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 2005; 76: 8–32.
Lettice LA, Hill RE : Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev 2005; 15: 294–300.
Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P : Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol 1999; 205: 79–97.
Griffin C, Kleinjan DA, Doe B, van Heyningen V : New 3′ elements control Pax6 expression in the developing pretectum, neural retina and olfactory region. Mech Dev 2002; 112: 89–100.
Benko S, Fantes JA, Amiel J et al: Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat Genet 2009; 41: 359–364.
Shaw CJ, Lupski JR : Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum Mol Genet 2004; 13 (Spec No 1): R57–R64.
Edelmann L, Spiteri E, Koren K et al: AT-rich palindromes mediate the constitutional t(11;22) translocation. Am J Hum Genet 2001; 68: 1–13.
Acknowledgements
C Durand is funded by a fellowship from Landesgraduiertenförderung Baden-Württemberg. We thank Ralph Roeth for technical assistance and valuable support and Katja Schneider for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Durand, C., Bangs, F., Signolet, J. et al. Enhancer elements upstream of the SHOX gene are active in the developing limb. Eur J Hum Genet 18, 527–532 (2010). https://doi.org/10.1038/ejhg.2009.216
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2009.216
Keywords
This article is cited by
-
Rare dosage abnormalities flanking the SHOX gene
Egyptian Journal of Medical Human Genetics (2021)
-
Identification of 15 novel partial SHOX deletions and 13 partial duplications, and a review of the literature reveals intron 3 to be a hotspot region
Journal of Human Genetics (2017)
-
Detection of SHOX gene aberrations in routine diagnostic practice and evaluation of phenotype scoring form effectiveness
Journal of Human Genetics (2017)
-
Systematic molecular analyses of SHOX in Japanese patients with idiopathic short stature and Leri–Weill dyschondrosteosis
Journal of Human Genetics (2016)
-
Rare pseudoautosomal copy-number variations involving SHOX and/or its flanking regions in individuals with and without short stature
Journal of Human Genetics (2015)