Abstract
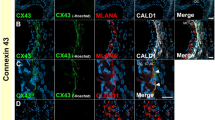
Hearing impairment is the most common sensory disorder worldwide. In a recent study, the authors have shown that a heterozygous missense mutation, p.R184Q, in the connexin 26 (Cx26) is causally related to hearing loss. However, the functional change in the Cx26R184Q mutant remains unknown. This study compared the intracellular distribution and assembly of mutant Cx26R184Q with that of the wild-type (WT) Cx26 and Cx30WT in tet-on HeLa cells and the effect that the mutant protein had on those cells. Fluorescent localization assay of WT Cx26 showed the typical punctuate pattern of gap junction channel between neighboring expression cells. Conversely, the p.R184Q missense mutation resulted in accumulation of the Cx26 mutant protein in the Golgi apparatus rather than in the cytoplasmic membrane. Cx26R184Q coexpressed with either Cx26WT or Cx30WT showed perinuclear localization by bidirectional tet-on expression system, suggesting the impairment of the ability of both WT proteins to intracellular trafficking and targeting to the plasma membrane. Therefore, we proposed that Cx26R184Q has a dominant-negative effect on the function of WT Cx26 and Cx30.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Apps SA, Rankin WA, Kurmis AP : Connexin26 mutations in autosomal recessive deafness disorders: a review. Int J Audiol 2007; 46: 75–81.
Morton NE : Genetic epidemiology of hearing impairment. Ann NY Acad Sci 1991; 630: 16–31.
Pallares-Ruiz N, Blanchet P, Mondain M, Claustres M, Roux AF : A large deletion including most of GJB6 in recessive nonsyndromic deafness: a digenic effect? Eur J Hum Genet 2002; 10: 72–76.
Morton CC : Genetics, genomics and gene discovery in the auditory system. Hum Mol Genet 2002; 11: 1229–1240.
Evans WH, Martin PE : Gap junctions: structure and function (review). Mol Membr Biol 2002; 19: 121–136.
Makowski L, Caspar DLD, Phillips WC, Goodenough DA : Gap junction structures. J Cell Biol 1997; 74: 629–645.
Willecke K, Eiberger J, Degen J et al: Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 2002; 383: 725–737.
Falk MM, Buehler LK, Kumar NM, Gilula NB : Cell-free synthesis of connexins into functional gap junction membrane channels. EMBO J 1997; 10: 2703–2716.
Musil LS, Goodenough DA : Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junction plaques. J Cell Biol 1991; 115: 1357–1374.
Falk MM, Kumar NM, Gilula NB : Membrane insertion of gap junction connexins: polytopic channel forming membrane proteins. J Cell Biol 1994; 127: 343–355.
Laird DW, Castillo M, Kasprzak L : Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in brefeldin A-treated rat mammary tumor cells. J Cell Biol 1995; 131: 1193–1203.
Sohl G, Willecke K : Gap junctions and the connexin protein family. Cardiovasc Res 2004; 62: 228–232.
Zhao HB, Kikuchi T, Ngezahayo A, White TW : Gap junctions and cochlear homeostasis. J Membr Biol 2006; 209: 177–186.
Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC : Gap junction systems in the mammalian cochlea. Brain Res 2000; 32: 163–166.
Ahmad S, Chen S, Sun J, Lin X : Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mouse. Bioche Biophy Res Comm 2003; 307: 362–368.
Marziano NK, Casalotti SO, Portelli AE, Becker DL, Forge A : Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet 2003; 12: 805–812.
Yang JJ, Huang SH, Chou KH, Liao PJ, Su CC, Li SY : Identification of mutations in members of connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol 2007; 12: 198–208.
Griffin BA, Adams SR, Tsien RY : Specific covalent labeling of recombinant protein molecules inside live cells. Science 1998; 281: 269.
Lautermann J, ten Cate WJ, Altenhoff P et al: Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 1998; 294: 415–420.
Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D’Andrea P : Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Comm 2003; 305: 1024–1033.
D’Andrea P, Veronesi V, Bicego M et al: Hearing loss: frequency and functional studies of the most common connexin26 alleles. Biochem Biophys Res Commun 2002; 296: 685–691.
Common JE, Becker D, Di WL, Leigh IM, O’Toole EA, Kelsell DP : Functional studies of human skin disease- and deafness-associated connexin 30 mutations. Biochem Biophys Res Commun 2002; 298: 651–656.
Thomas T, Jordan K, Simek J et al: Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci 2005; 118: 4451–4462.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H : Transcriptional activation by tetracycline in mammalian cells. Science 1995; 268: 1766–1769.
Azaiez H, Chamberlin GP, Fischer SM et al: GJB2: the spectrum of deafness-causing allele variants and their phenotype. Hum Mutat 2004; 24: 305–311.
Hamelmann C, Amedofu GK, Albrecht K et al: Pattern of connexin 26 (GJB2) mutations causing sensorineural hearing impairment in Ghana. Hum Mutat 2001; 18: 84–85.
Krutovskikh V, Yamasaki H : Connexin gene mutations in human genetic diseases. Muta Res 2000; 462: 197–207.
Berezin C, Glaser F, Rosenberg J et al: ConSeq: the identification of functionally and structurally important residues in protein sequences. Bioinformatics 2004; 20: 1322–1324.
Wilcox SA, Saunders K, Osborn AH et al: High frequency hearing loss correlated with mutations in the GJB2 gene. Hum Genet 2000; 106: 399–405.
Denoyelle F, Weil D, Maw MA et al: Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet 1997; 6: 2173–2177.
Evans WH, Ahmad S, Diez J, George CH, Kendall JM, Martin PE : Trafficking pathways leading to the formation of gap junctions. Novartis Found Symp 1999; 219: 44–54.
Qu C, Gardner P, Schrijver I : The role of the cytoskeleton in the formation of gap junctions by connexin 30. Exp Cell Res 2009; 315: 1683–1692.
Acknowledgements
We thank the Chung Shan Medical University, Tian-Sheng Memorial Hospital (CSMU-TSMH-097-001) and the National Science Council of the Republic of China, Taiwan for financially supporting this research under Contract No. NSC98-2320-B-040-016-MY3. Ted Knoy is appreciated for the editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Su, CC., Li, SY., Su, MC. et al. Mutation R184Q of connexin 26 in hearing loss patients has a dominant-negative effect on connexin 26 and connexin 30. Eur J Hum Genet 18, 1061–1064 (2010). https://doi.org/10.1038/ejhg.2010.50
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2010.50
Keywords
This article is cited by
-
Roles of aberrant hemichannel activities due to mutant connexin26 in the pathogenesis of KID syndrome
Scientific Reports (2018)
-
Rare compound heterozygosity involving dominant and recessive mutations of GJB2 gene in an assortative mating hearing impaired Indian family
European Archives of Oto-Rhino-Laryngology (2017)
-
Altered cellular localization and hemichannel activities of KID syndrome associated connexin26 I30N and D50Y mutations
BMC Cell Biology (2016)
-
Mechanisms linking connexin mutations to human diseases
Cell and Tissue Research (2015)
-
Human Connexin30.2/31.3 (GJC3) does not Form Functional Gap Junction Channels but Causes Enhanced ATP Release in HeLa Cells
Cell Biochemistry and Biophysics (2011)