Abstract
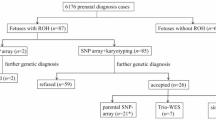
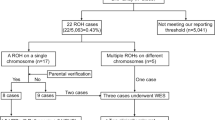
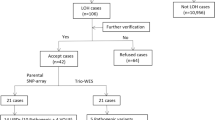
We report on the validation and implementation of the HumanCytoSNP-12 array (Illumina) (HCS) in prenatal diagnosis. In total, 64 samples were used to validate the Illumina platform (20 with a known (sub) microscopic chromosome abnormality, 5 with known maternal cell contamination (MCC) and 39 normal control samples). There were no false-positive or false-negative results. In addition to the diagnostic possibilities of arrayCGH, the HCS allows detection of regions of homozygosity (ROH), triploidy and helps recognising MCC. Moreover, in two cases of MCC, a deletion was correctly detected. Furthermore we found out that only about 50 ng of DNA is required, which allows a reporting time of only 3 days. We also present a prospective pilot study of 61 fetuses with ultrasound abnormalities and a normal karyotype tested with HCS. In 4 out of 61 (6.5%) fetuses, a clinically relevant abnormality was detected. We designed and present pre-test genetic counselling information on categories of possible test outcomes. On the basis of this information, about 90% of the parents chose to be informed about adverse health outcomes of their future child at infancy and childhood, and 55% also about outcomes at an adult stage. The latter issue regarding the right of the future child itself to decide whether or not to know this information needs to be addressed.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Shaffer LG, Coppinger J, Alliman S et al: Comparison of microarray-based detection rates for cytogenetic abnormalities in prenatal and neonatal specimens. Prenat Diagn 2008; 28: 789–795.
Rickman L, Fiegler H, Carter NP, Bobrow M : Prenatal diagnosis by array-CGH. Eur J Med Genet 2005; 48: 232–240.
Rickman L, Fiegler H, Shaw-Smith C et al: Prenatal detection of unbalanced chromosomal rearrangements by array CGH. J Med Genet 2006; 43: 353–361.
Sahoo T, Cheung SW, Ward P et al: Prenatal diagnosis of chromosomal abnormalities using array-based comparative genomic hybridization. Genet Med 2006; 8: 719–727.
Friedman JM : High resolution array genomic hybridization in prenatal diagnosis. Prenat Diagn 2009; 29: 20–28.
Kleeman L, Bianchi D, Shaffer LG et al: Use of array comparative genomic hybridization for prenatal diagnosis of foetuses with sonographic anomalies and normal metaphase karyotype. Prenat Diagn 2009; 29: 1213–1217.
Van den Veyver IB, Patel A, Shaw CA et al: Clinical use of array comparative genomic hybridization (aCGH) for prenatal diagnosis in 300 cases. Prenat Diagn 2009; 29: 29–39.
Tyreman M, Abbott KM, Willatt LR et al: High resolution array analysis: diagnosing pregnancies with abnormal ultrasound findings. J Med Genet 2009; 46: 531–541.
Faas BH, van der Burgt I, Kooper AJ et al: Identification of clinically significant, submicroscopic chromosome alterations and UPD in foetuses with ultrasound anomalies using genome-wide 250 k SNP array analysis. J Med Genet 2010; 47: 586–594.
Vermeesch JR, Fiegler H, de Leeuw N : Guidelines for molecular karyotyping in constitutional genetic diagnosis. Eur J Hum Genet 2007; 15: 1105–1114.
Murthy SK, Nygren AO, El Shakankiry HM et al: Detection of a novel familial deletion of four genes between BP1 and BP2 of the Prader-Willi/Angelman syndrome critical region by oligo-array CGH in a child with neurological disorder and speech impairment. Cytogenet Genome Res 2007; 116: 135–140.
Doornbos M, Sikkema-Raddatz B, Ruijvenkamp CA et al: Nine patients with a microdeletion 15q11.2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbances. Eur J Med Genet 2009; 52: 108–115.
de Kovel CG, Trucks H, Helbig I et al: Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 2010; 133 (part 1): 23–32.
Lecointre C, Pichon O, Hamel A et al: Familial acampomelic form of campomelic dysplasia caused by a 960 kb deletion upstream of SOX9. Am J Med Genet A 2009; 149A: 1183–1189.
Kurahashi H, Nakayama T, Osugi Y et al: Deletion mapping of 22q11 in CATCH22 syndrome: identification of a second critical region. Am J Hum Genet 1996; 58: 1377–1381.
Talseth-Palmer BA, Bowden NA, Hill A, Meldrum C, Scott RJ : Whole genome amplification and its impact on CGH array profiles. BMC Res Notes 2008; 1: 56.
Le Caignec C, Boceno M, Saugier-Veber P et al: Detection of genomic imbalances by array based comparative genomic hybridisation in foetuses with multiple malformations. J Med Genet 2005; 42: 121–128.
Valduga M, Philippe C, Bach Segura P et al: A retrospective study by oligonucleotide array-CGH analysis in 50 foetuses with multiple malformations. Prenat Diagn 2010; 30: 333–341.
Hillman SC, Pretlove S, Coomarasamy A et al: Additional information from array comparative genomic hybridisation (array CGH) technology over conventional karyotyping in prenatal diagnosis-a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2011; 37: 6–14.
Veltman JA, de Vries BB : Diagnostic genome profiling: unbiased whole genome or targeted analysis? J Mol Diagn 2006; 8: 534–539.
Coppinger J, Alliman S, Lamb AN, Torchia BS, Bejjani BA, Shaffer LG : Whole-genome microarray analysis in prenatal specimens identifies clinically significant chromosome alterations without increase in results of unclear significance compared to targeted microarray. Prenat Diagn 2009; 29: 1156–1166.
Vissers LELM, de Vries BBA, Veltman JA : Genomic microarrays in mental retardation: from copy number variation to gene, from research to diagnosis. J Med Genet 2010; 47: 289–297.
Conlin LK, Thiel BD, Bonnemann CG et al: Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum Mol Genet 2010; 19: 1263–1275.
van de Vathorst S, Verhagen AA, Wildschut HI, Wolf H, Zeeman GG, Lind J : Termination of pregnancy after the 20-week ultrasonographic examination: haste and caution. Ned Tijdschr Geneeskd 2008; 152: 2589–2591.
Acknowledgements
We thank B Beverloo and J Saris for using the array facility unit, Y van Bever for referring cases and gynaecologists for referring cases to the clinical geneticists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Srebniak, M., Boter, M., Oudesluijs, G. et al. Application of SNP array for rapid prenatal diagnosis: implementation, genetic counselling and diagnostic flow. Eur J Hum Genet 19, 1230–1237 (2011). https://doi.org/10.1038/ejhg.2011.119
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2011.119
Keywords
This article is cited by
-
Optimizing the Diagnostic Strategy to Identify Genetic Abnormalities in Miscarriage
Molecular Diagnosis & Therapy (2021)
-
Clinical experience with multiplex ligation-dependent probe amplification for microdeletion syndromes in prenatal diagnosis: 7522 pregnant Korean women
Molecular Cytogenetics (2019)
-
Choosing between Higher and Lower Resolution Microarrays: do Pregnant Women Have Sufficient Knowledge to Make Informed Choices Consistent with their Attitude?
Journal of Genetic Counseling (2018)
-
Enlarged NT (≥3.5 mm) in the first trimester – not all chromosome aberrations can be detected by NIPT
Molecular Cytogenetics (2016)
-
Prenatal SNP array testing in 1000 fetuses with ultrasound anomalies: causative, unexpected and susceptibility CNVs
European Journal of Human Genetics (2016)