Abstract
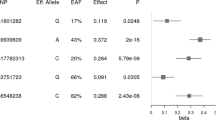
Genome-wide association studies have shown a strong association of single-nucleotide polymorphisms (SNPs) in the near vicinity of the TMEM18 gene. The effects of the TMEM18-associated variants are more readily observed in children. TMEM18 encodes a 3TM protein, which locates to the nuclear membrane. The functional context of TMEM18 and the effects of its associated variants are as of yet undetermined. To further explore the effects of near-TMEM18 variants, we have genotyped two TMEM18-associated SNPs, rs6548238 and rs4854344, in a cohort of 2352 Greek children (Healthy Growth Study). Included in this study are data on anthropomorphic traits body weight, BMI z-score and waist circumference. Also included are dietary energy and macronutrient intake as measured via 24-h recall interviews. Major alleles of rs6548238 and rs4854344 were significantly associated with an increased risk of obesity (odds ratio=1.489 (1.161–1.910) and 1.494 (1.165–1.917), respectively), and positively correlated to body weight (P=0.017, P=0.010) and waist circumference (P=0.003, P=0.003). An association to energy and macronutrient intake was not observed in this cohort. We also correlated food intake and body weight in a food choice model in rats to Tmem18 expression in central regions involved in feeding behavior. We observed a strong positive correlation between TMEM18 expression and body weight in the prefrontal cortex (PFC) (r=0.5694, P=0.0003) indicating a potential role for TMEM18 in higher functions related to feeding involving the PFC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Willer CJ, Speliotes EK, Loos RJ et al: Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009; 41: 25–34.
Speliotes EK, Willer CJ, Berndt SI et al: Association analyses of 249 796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010; 42: 937–948.
Frayling TM, Timpson NJ, Weedon MN et al: A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007; 316: 889–894.
Fredriksson R, Hagglund M, Olszewski PK et al: The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008; 149: 2062–2071.
Olszewski PK, Fredriksson R, Olszewska AM et al: Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci 2009; 10: 129.
Church C, Moir L, McMurray F et al: Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010; 42: 1086–1092.
Berulava T, Horsthemke B : The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet 2010; 18: 1054–1056.
Thorleifsson G, Walters GB, Gudbjartsson DF et al: Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009; 41: 18–24.
Zhao J, Bradfield JP, Li M et al: The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity (Silver Spring) 2009; 17: 2254–2257.
den Hoed M, Ekelund U, Brage S et al: Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes 2010; 59: 2980–2988.
Almen MS, Jacobsson JA, Shaik JH et al: The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. BMC Med Genet 2010; 11: 58.
Renstrom F, Payne F, Nordstrom A et al: Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet 2009; 18: 1489–1496.
Ng MC, Tam CH, So WY et al: Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab 2010; 95: 2418–2425.
Hotta K, Nakamura M, Nakamura T et al: Association between obesity and polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese population. J Hum Genet 2009; 54: 727–731.
Jurvansuu J, Zhao Y, Leung DS et al: Transmembrane protein 18 enhances the tropism of neural stem cells for glioma cells. Cancer Res 2008; 68: 4614–4622.
Lindblom J, Haitina T, Fredriksson R, Schioth HB : Differential regulation of nuclear receptors, neuropeptides and peptide hormones in the hypothalamus and pituitary of food restricted rats. Brain Res Mol Brain Res 2005; 133: 37–46.
Moschonis G, Tanagra S, Vandorou A et al: Social, economic and demographic correlates of overweight and obesity in primary-school children: preliminary data from the Healthy Growth Study. Public Health Nutr 2010; 13: 1693–1700.
Jacobsson JA, Rask-Andersen M, Riserus U et al: Genetic variants near the MGAT1 gene are associated with body weight, BMI and fatty acid metabolism among adults and children. Int J Obes (Lond) 2011; e-pub ahead of print 8 February 2011; doi:10.1038/ijo.2011.11.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH : Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (Clinical research ed.) 2000; 320: 1240–1243.
Schofield WN : Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985; 39 (Suppl 1): 5–41.
Goldberg GR, Black AE, Jebb SA et al: Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991; 45: 569–581.
Trichopoulou A : Composition Tables of Foods and Greek Dishes. 3rd Edn. Parisianou Publications: Athens, Greece, 2004.
Barrett JC, Fry B, Maller J, Daly MJ : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Gabriel SB, Schaffner SF, Nguyen H et al: The structure of haplotype blocks in the human genome. Science 2002; 296: 2225–2229.
Purcell S, Neale B, Todd-Brown K et al: PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Benjamini Y, Hochberg Y : Controlling the false discovery rate – a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995; 57: 289–300.
Skol AD, Scott LJ, Abecasis GR, Boehnke M : Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet 2006; 38: 209–213.
Alsiö J, Roman E, Olszewski PK et al: Inverse association of high-fat diet preference and anxiety-like behavior: a putative role for urocortin 2. Genes Brain Behav 2009; 8: 193–202.
Rio DC, Ares M, Hannon GJ, Nilsen TW : Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protoc 2010, pdb prot5439.
Vandesompele J, De Preter K, Pattyn F et al: Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: RESEARCH0034.
Elks CE, Loos RJ, Sharp SJ et al: Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med 2010; 7: e1000284.
Kilpelainen TO, den Hoed M, Ong KK et al: Obesity-susceptibility loci have a limited influence on birth weight: a meta-analysis of up to 28 219 individuals. Am J Clin Nutr 2011; 93: 851–860.
Miller EK, Cohen JD : An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202.
Kuboshima-Amemori S, Sawaguchi T : Plasticity of the primate prefrontal cortex. Neuroscientist 2007; 13: 229–240.
Goto Y, Yang CR, Otani S : Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry 2010; 67: 199–207.
Amargos-Bosch M, Adell A, Bortolozzi A, Artigas F : Stimulation of alpha1-adrenoceptors in the rat medial prefrontal cortex increases the local in vivo 5-hydroxytryptamine release: reversal by antipsychotic drugs. J Neurochem 2003; 87: 831–842.
Andrade R : Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology 2011; 61: 382–386.
Keramidas A, Harrison NL : The activation mechanism of alpha1beta2gamma2S and alpha3beta3gamma2S GABAA receptors. J Gen Physiol 2010; 135: 59–75.
Yee BK, Keist R, von Boehmer L et al: A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA 2005; 102: 17154–17159.
Acknowledgements
The study was supported by the Swedish Research Council, Brain Research Foundation, Novo Nordisk, Tore Nilsons foundation and Åhlens foundation. RF was supported by the Göran Gustafsson foundation. The SNP genotyping was performed by the SNP Technology Platform, Uppsala, Sweden (http://www.genotyping.se) with support from Uppsala University and the Knut and Alice Wallenberg foundation, and at the Uppsala Genome Centre. We thank the Healthy Growth Study Group for their contribution in this study. The Healthy Growth Study Group consists of (1) Harokopio University Research Team/Department of Nutrition and Dietetics: Yannis Manios (Coordinator), George Moschonis (Project manager), Katerina P Skenderi, Evangelia Grammatikaki, Odysseas Androutsos, Sofia Tanagra, Alexandra Koumpitski, Paraskevi-Eirini Siatitsa, Anastasia Vandorou, Aikaterini-Efstathia Kyriakou, Vasiliki Dede, Maria Kantilafti, Aliki-Eleni Farmaki, Aikaterini Siopi, Sofia Micheli, Louiza Damianidi, Panagiota Margiola, Despoina Gakni, Vasiliki Iatridi, Christina Mavrogianni, Kelaidi Michailidou, Aggeliki Giannopoulou, Efstathoula Argyri, Konstantina Maragkopoulou, Maria Spyridonos, Eirini Tsikalaki, Panagiotis Kliasios, Anthi Naoumi, Konstantinos Koutsikas, Katerina Kondaki, Epistimi Aggelou, Zoi Krommyda, Charitini Aga, Manolis Birbilis, Ioanna Kosteria, Amalia Zlatintsi, Elpida Voutsadaki, Eleni-Zouboulia Papadopoulou, Zoi Papazi, Maria Papadogiorgakaki, Fanouria Chlouveraki, Maria Lyberi, Nora Karatsikaki-Vlami, Eva Dionysopoulou and Efstratia Daskalou. (2) Aristotle University of Thessaloniki/School of Physical Education and Sports Sciences: Vassilis Mougios, Anatoli Petridou, Konstantinos Papaioannou, Georgios Tsalis, Ananis Karagkiozidis, Konstantinos Bougioukas, Afroditi Sakellaropoulou and Georgia Skouli. (3) University of Athens/ Medical School: George P Chrousos, Maria Drakopoulou and Evangelia Charmandari.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Rask-Andersen, M., Jacobsson, J., Moschonis, G. et al. Association of TMEM18 variants with BMI and waist circumference in children and correlation of mRNA expression in the PFC with body weight in rats. Eur J Hum Genet 20, 192–197 (2012). https://doi.org/10.1038/ejhg.2011.176
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2011.176
Keywords
This article is cited by
-
Interaction between genetic susceptibility to obesity and food intake on BMI in Finnish school-aged children
Scientific Reports (2023)
-
The association between polymorphisms near TMEM18 and the risk of obesity: a meta-analysis
BMC Medical Genomics (2021)
-
Association of the Variant rs7561317 Downstream of the TMEM18 Gene with Overweight/Obesity and Related Anthropometric Traits in a Sample of Pakistani Population
Biochemical Genetics (2020)
-
Obesity-Related Genetic Variants and their Associations with Physical Activity
Sports Medicine - Open (2015)
-
Genetics of Obesity
Current Obesity Reports (2013)