Abstract
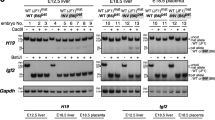
ART is suspected to generate increased imprinting errors in the lineage. Following an intra cytoplasmic sperm injection (ICSI) procedure, a certain number of embryos fail to develop normally and imprinting disorders may be associated to the developmental failure. To evaluate this hypothesis, we analysed the methylation profile of H19DMR, a paternally imprinting control region, in high-graded blastocysts, in embryos showing developmental anomalies, in the matching sperm and in oocytes of the concerned couples when they were available. Significant hypomethylation of the paternal allele was observed in half of the embryos, independently of the stage at which they were arrested (morula, compacted morula, pre blastocyst or BC-graded blastocysts). Conversely, some embryos showed significant methylation on the maternal allele, whereas few others showed both hypomethylation of the paternal allele and abnormal methylation of the maternal allele. The matching sperm at the origin of the embryos exhibited normal methylated H19 patterns. Thus, hypomethylation of the paternal allele in the embryos does not seem inherited from the sperm but likely reflects instability of the imprint during the demethylating process, which occurred in the early embryo. Analysis of a few oocytes suggests that the defect in erasure of the paternal imprint in the maternal germ line may be responsible for the residual methylation of the maternal allele in some embryos. None of these imprinting alterations could be related to a particular stage of developmental arrest; compared with high-grade blastocysts, embryos with developmental failure are more likely to have abnormal imprinting at H19 (P<0.05).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Surani MA : Imprinting and the initiation of gene silencing in the germ line. Cell 1998; 93: 309–312.
Hajkova P, Erhardt S, Lane N et al: Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 2002; 117: 15–23.
Reik W, Dean W, Walter J : Epigenetic reprogramming in mammalian development. Science 2001; 293: 1089–1093.
DeBaun MR, Niemitz EL, Feinberg AP : Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003; 72: 156–160.
Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc Y : In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet 2003; 72: 1338–1341.
Maher ER, Brueton LA, Bowdin SC et al: Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet 2003; 40: 62–64.
Orstavik KH, Eiklid K, van der Hagen CB et al: Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet 2003; 72: 218–219.
Bowdin S, Allen C, Kirby G et al: A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod 2007; 22: 3237–3240.
Grace KS, Sinclair KD : Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med 2009; 27: 409–416.
Zemel S, Bartolomei MS, Tilghman SM : Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat Genet 1992; 2: 61–65.
Yoshimizu T, Miroglio A, Ripoche MA et al: The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci USA 2008; 105: 12417–12422.
Reik W, Constancia M, Dean W et al: Igf2 imprinting in development and disease. Int J Dev Biol 2000; 44: 145–150.
Takai D, Gonzales FA, Tsai YC, Thayer MJ, Jones PA : Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum Mol Genet 2001; 10: 2619–2626.
Gicquel C, Rossignol S, Cabrol S et al: Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat Genet 2005; 37: 1003–1007.
Borghol N, Lornage J, Blachere T, Garret AS, Lefèvre A : Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics 2006; 87: 417–426.
Santos F, Hendrich B, Reik W, Dean W : Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 2002; 241: 172–182.
Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR : Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet 2010; 19: 36–51.
Kobayashi H, Hiura H, John RM et al: DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet 2009; 17: 1582–1591.
Chopra M, Amor DJ, Sutton L, Algar E, Mowat D : Russell-Silver syndrome due to paternal H19/IGF2 hypomethylation in a patient conceived using intracytoplasmic sperm injection. Reprod Biomed Online 2010; 20: 843–847.
Renda M, Baglivo I, Burgess-Beusse B et al: Critical DNA binding interactions of the insulator protein CTCF: a small number of zinc fingers mediate strong binding, and a single finger-DNA interaction controls binding at imprinted loci. J Biol Chem 2007; 282: 33336–33345.
Boissonnas CC, Abdalaoui HE, Haelewyn V et al: Specific epigenetic alterations of IGF2-H19 locus in spermatozoa from infertile men. Eur J Hum Genet 2010; 18: 73–80.
Marques CJ, Costa P, Vaz B et al: Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol Hum Reprod 2008; 14: 67–74.
Poplinski A, Tuttelmann F, Kanber D, Horsthemke B, Gronoll J : Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl 2010; 33: 642–649.
Hirasawa R, Chiba H, Kaneda M et al: Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev 2008; 22: 1607–1616.
Reese KJ, Lin S, Verona RI, Schultz RM, Bartolomei MS : Maintenance of paternal methylation and repression of the imprinted H19 gene requires MBD3. PLoS Genet 2007; 3: e137.
Acknowledgements
We are very grateful to all the couples that donated embryos and gametes for research and to the staff of ‘Service de Biologie de la Reproduction’ at FME Hospital, Bron, particularly to Jacqueline Lornage and Astrid Perret. This work was supported by Université Claude Bernard Lyon 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Ibala-Romdhane, S., Al-Khtib, M., Khoueiry, R. et al. Analysis of H19 methylation in control and abnormal human embryos, sperm and oocytes. Eur J Hum Genet 19, 1138–1143 (2011). https://doi.org/10.1038/ejhg.2011.99
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2011.99
Keywords
This article is cited by
-
Cloning and expression analysis of GATA1 gene in Carassius auratus red var
BMC Genomic Data (2021)
-
Loss of methylation of H19-imprinted gene derived from assisted reproductive technologies can be mitigated by cleavage-stage embryo transfer in mice
Journal of Assisted Reproduction and Genetics (2019)
-
Differential expression of parental alleles of BRCA1 in human preimplantation embryos
European Journal of Human Genetics (2017)
-
High Frequency of Imprinted Methylation Errors in Human Preimplantation Embryos
Scientific Reports (2015)
-
Epigenetische Aspekte der Reproduktionsmedizin
Gynäkologische Endokrinologie (2014)