Abstract
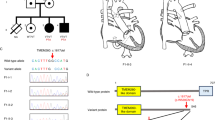
GJA5 gene (MIM no. 121013), localized at 1q21.1, encodes for the cardiac gap junction protein connexin 40. In humans, copy number variants of chromosome 1q21.1 have been associated with variable phenotypes comprising congenital heart disease (CHD), including isolated TOF. In mice, the deletion of Gja5 can cause a variety of complex CHDs, in particular of the cardiac outflow tract, corresponding to TOF in many cases. In the present study, we screened for mutations in the GJA5 gene 178 unrelated probands with isolated TOF. A heterozygous nucleotide change (c.793C>T) in exon 2 of the gene leading to the p.Pro265Ser variant at the carboxyl-terminus of the protein was found in two unrelated sporadic patients, one with classic anatomy and one with pulmonary atresia. This GJA5 missense substitution was not observed in 1568 ethnically-matched control chromosomes. Immunofluorescent staining and confocal microscopy revealed that cells expressing the mutant protein form sparse or no visible gap-junction plaques in the region of cell–cell contact. Moreover, analysis of the transfer of the gap junction permanent tracer lucifer yellow showed that cells expressing the mutant protein have a reduced rate of dye transfer compared with wild-type cells. Finally, use of a zebrafish model revealed that microinjection of the GJA5-p.Pro265Ser mutant disrupts overall morphology of the heart tube in the 37% (22/60) of embryos, compared with the 6% (4/66) of the GJA5 wild-type-injected embryos. These findings implicate GJA5 gene as a novel susceptibility gene for TOF.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Ferencz C, Rubin JD, McCarter RJ et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 1985; 121: 31–36.
Amati F, Mari A, Digilio MC et al. 22q11 deletions in isolated and syndromic patients with tetralogy of Fallot. Hum Genet 1995; 95: 479–482.
Goldmuntz E, Clark BJ, Mitchell LE et al. Frequency of 22q11 deletions in patients with conotruncal defects. J Am Coll Cardiol 1998; 32: 492–498.
Goldmuntz E, Geiger E, Benson DW : NKX2.5 mutations in patients with tetralogy of fallot. Circulation 2001; 104: 2565–2568.
McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E : NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol 2003; 42: 1650–1655.
Nemer G, Fadlalah F, Usta J et al. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat 2006; 27: 293–294.
Tomita-Mitchell A, Maslen CL, Morris CD, Garg V, Goldmuntz E : GATA4 sequence variants in patients with congenital heart disease. J Med Genet 2007; 44: 779–783.
De Luca A, Sarkozy A, Ferese R et al. New mutations in ZFPM2/FOG2 gene in tetralogy of Fallot and double outlet right ventricle. Clin Genet 2011; 80: 184–190.
Roessler E, Ouspenskaia MV, Karkera JD et al. Reduced NODAL signaling strength via mutation of several pathway members including FOXH1 is linked to human heart defects and holoprosencephaly. Am J Hum Genet 2008; 83: 18–29.
Garg V, Muth AN, Ransom JF et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005; 437: 270–274.
Eldadah ZA, Hamosh A, Biery NJ et al. Familial Tetralogy of Fallot caused by mutation in the jagged1 gene. Hum Mol Genet 2001; 10: 163–169.
Guida V, Chiappe F, Ferese R et al. Novel and recurrent JAG1 mutations in patients with tetralogy of Fallot. Clin Genet 2011; 80: 591–594.
Karkera JD, Lee JS, Roessler E et al. Loss-of-function mutations in growth differentiation factor-1 (GDF1) are associated with congenital heart defects in humans. Am J Hum Genet 2007; 81: 987–994.
Roessler E, Pei W, Ouspenskaia MV et al. Cumulative ligand activity of NODAL mutations and modifiers are linked to human heart defects and holoprosencephaly. Mol Genet Metab 2009; 98: 225–234.
Schott JJ, Benson DW, Basson CT et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998; 281: 108–111.
Garg V, Kathiriya IS, Barnes R et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 2003; 424: 443–447.
De Luca A, Sarkozy A, Consoli F et al. Familial transposition of the great arteries caused by multiple mutations in laterality genes. Heart 2010; 96: 673–677.
Greenway SC, Pereira AC, Lin JC et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat Genet 2009; 41: 931–935.
Soemedi R, Topf A, Wilson IJ et al. Phenotype-specific effect of chromosome 1q21.1 rearrangements and GJA5 duplications in 2436 congenital heart disease patients and 6760 controls. Hum Mol Genet 2012; 21: 1513–1520.
Gu H, Smith FC, Taffet SM, Delmar M : High incidence of cardiac malformations in connexin40-deficient mice. Circ Res 2003; 93: 201–206.
Gollob MH, Jones DL, Krahn AD et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med 2006; 354: 2677–2688.
Yang YQ, Liu X, Zhang XL et al. Novel connexin40 missense mutations in patients with familial atrial fibrillation. Europace 2010; 12: 1421–1427.
Yang YQ, Zhang XL, Wang XH et al. Connexin40 nonsense mutation in familial atrial fibrillation. Int J Mol Med 2010; 26: 605–610.
Westerfield M : The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). University of Oregon Press: Eugene, OR, 1995.
Thisse C, Thisse B, Schilling TF, Postlethwait JH : Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 1993; 119: 1203–1215.
Yelon D, Horne SA, Stainier DY : Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 1999; 214: 23–37.
Gros D, Dupays L, Alcolea S, Meysen S, Miquerol L, Theveniau-Ruissy M : Genetically modified mice: tools to decode the functions of connexins in the heart-new models for cardiovascular research. Cardiovasc Res 2004; 62: 299–308.
Pfenniger A, Wohlwend A, Kwak BR : Mutations in connexin genes and disease. Eur J Clin Invest 2011; 41: 103–116.
Sarkozy A, Conti E, Neri C et al. Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. J Med Genet 2005; 42: e16.
Rauch R, Hofbeck M, Zweier C et al. Comprehensive genotype-phenotype analysis in 230 patients with tetralogy of Fallot. J Med Genet 2010; 47: 321–331.
Brunetti-Pierri N, Berg JS, Scaglia F et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet 2008; 40: 1466–1471.
Mefford HC, Eichler EE : Duplication hotspots, rare genomic disorders, and common disease. Curr Opin Genet Dev 2009; 19: 196–204.
Mefford HC, Sharp AJ, Baker C et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med 2008; 359: 1685–1699.
Christiansen J, Dyck JD, Elyas BG et al. Chromosome 1q21.1 contiguous gene deletion is associated with congenital heart disease. Circ Res 2004; 94: 1429–1435.
Erdogan F, Larsen LA, Zhang L et al. High frequency of submicroscopic genomic aberrations detected by tiling path array comparative genome hybridisation in patients with isolated congenital heart disease. J Med Genet 2008; 45: 704–709.
Digilio MC, Marino B, Grazioli S, Agostino D, Giannotti A, Dallapiccola B : Comparison of occurrence of genetic syndromes in ventricular septal defect with pulmonic stenosis (classic tetralogy of Fallot) versus ventricular septal defect with pulmonic atresia. Am J Cardiol 1996; 77: 1375–1376.
Marino B, Digilio MC, Toscano A et al. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet Med 2001; 3: 45–48.
van Veen AA, van Rijen HV, Opthof T : Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 2001; 51: 217–229.
Kirchhoff S, Kim JS, Hagendorff A et al. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ Res 2000; 87: 399–405.
Bouvier D, Kieken F, Kellezi A, Sorgen PL : Structural changes in the carboxyl terminus of the gap junction protein connexin 40 caused by the interaction with c-Src and zonula occludens-1. Cell Commun Adhes 2008; 15: 107–118.
Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z : Intrinsic disorder and protein function. Biochemistry 2002; 41: 6573–6582.
Dunker AK, Obradovic Z : The protein trinity – linking function and disorder. Nat Biotechnol 2001; 19: 805–806.
Zhou L, Kasperek EM, Nicholson BJ : Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol 1999; 144: 1033–1045.
Lin R, Warn-Cramer BJ, Kurata WE, Lau AF : v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol 2001; 154: 815–827.
Jia CY, Nie J, Wu C, Li C, Li SS : Novel Src homology 3 domain-binding motifs identified from proteomic screen of a Pro-rich region. Mol Cell Proteomics 2005; 4: 1155–1166.
Duffy HS, Ashton AW, O’Donnell P et al. Regulation of connexin43 protein complexes by intracellular acidification. Circ Res 2004; 94: 215–222.
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M : Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 1998; 273: 12725–12731.
Hunter AW, Barker RJ, Zhu C, Gourdie RG : Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 2005; 16: 5686–5698.
Barker RJ, Price RL, Gourdie RG : Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 2002; 90: 317–324.
Acknowledgements
The authors wish to thank the patients who participated in this research. We especially thank Dr MH Gollob for kindly providing the full-length human wild-type GJA5 cDNA. This work was supported in part by the Italian Ministry of Health grant RC2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Guida, V., Ferese, R., Rocchetti, M. et al. A variant in the carboxyl-terminus of connexin 40 alters GAP junctions and increases risk for tetralogy of Fallot. Eur J Hum Genet 21, 69–75 (2013). https://doi.org/10.1038/ejhg.2012.109
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2012.109
Keywords
This article is cited by
-
Genetic and ultrasonographic analyses of fetuses with 1q21.1q21.2 microdeletion/microduplication: a retrospective study
BMC Medical Genomics (2023)
-
Detection of copy number variation associated with ventriculomegaly in fetuses using single nucleotide polymorphism arrays
Scientific Reports (2021)
-
Parental Occupational Exposures to Endocrine Disruptors and the Risk of Simple Isolated Congenital Heart Defects
Pediatric Cardiology (2015)
-
Association of promoter methylation statuses of congenital heart defect candidate genes with Tetralogy of Fallot
Journal of Translational Medicine (2014)
-
DNA methylation status of NKX2-5, GATA4 and HAND1in patients with tetralogy of fallot
BMC Medical Genomics (2013)