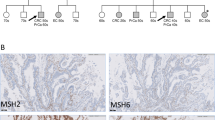
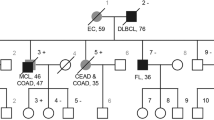
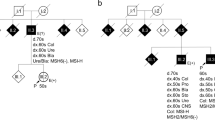
Abstract
Lynch syndrome is an autosomal-dominant hereditary condition predisposing to the development of specific cancers, because of germline mutations in the DNA-mismatch repair (MMR) genes. Large genomic deletions represent a significant fraction of germline mutations, particularly among the MSH2 gene, in which they account for 20% of the mutational spectrum. In this study we analyzed 13 Italian families carrying MSH2 exon 8 deletions, 10 of which of ascertained Sardinian origin. The overrepresentation of Sardinians was unexpected, as families from Sardinia account for a small quota of MMR genes mutation tests performed in our laboratory. The hypothesis that such a result is owing to founder effects in Sardinia was tested by breakpoint junctions sequencing and haplotype analyses. Overall, five different exon eight deletions were identified, two of which recurrent in families, all apparently unrelated, of Sardinian origin (one in eight families, one in two families). The c.1277–1180_1386+2226del3516insCATTCTCTTTGAAAA deletion shares the same haplotype between all families and appears so far restricted to the population of South-West Sardinia, showing the typical features of a founder effect. The three non-Sardinian families showed three different breakpoint junctions and haplotypes, suggesting independent mutational events. This work has useful implications in genetic testing for Lynch syndrome. We developed a quick test for each of the identified deletions: this can be particularly useful in families of Sardinian origin, in which MSH2 exon 8 deletions may represent 50% of the overall mutational spectrum of the four MMR genes causing Lynch syndrome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vasen HF, Watson P, Mecklin JP, Lynch HT : New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999; 116: 1453–1456.
Umar A, Boland CR, Terdiman JP et al. Revised Bethesda guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96: 261–268.
van der Klift H, Wijnen J, Wagner A et al. Molecular characterization of the spectrum of genomic deletions in the mismatch repair genes MSH2, MLH1, MSH6, and PMS2 responsible for hereditary nonpolyposis colorectal cancer (HNPCC). Genes Chromosomes Cancer 2005; 44: 123–138.
Thiffault I, Hamel N, Pal T et al. Germline truncating mutations in both MSH2 and BRCA2 in a single kindred. Br J Cancer 2004; 90: 483–491.
Stella A, Surdo NC, Lastella P et al. Germline novel MSH2 deletions and a founder MSH2 deletion associated with anticipation effects in HNPCC. Clin Genet 2007; 71: 130–139.
Gille JJ, Hogervorst FB, Pals G et al. Genomic deletions of MSH2 and MLH1 in colorectal cancer families detected by a novel mutation detection approach. Br J Cancer 2002; 87: 892–897.
Nakagawa H, Hampel H, de la Chapelle A : Identification and characterization of genomic rearrangements of MSH2 and MLH1 in Lynch Syndrome (HNPCC) by Novel Techniques. Hum Mutat 2003; 22: 258–263.
Clendenning M, Baze ME, Sun S et al. Origins and Prevalence of the American Founder Mutation of MSH2. Cancer Res 2008; 68: 2145–2153.
Ruszkiewicz A, Bennett G, Moore J et al. Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumours. Pathology 2002; 34: 541–547.
Hansen TP, Nielsen O, Fenger C : Optimization of antibodies for detection of the mismatch repair proteins MLH1, MSH2, MSH6, and MS2 using a biotin-free visualization system. Appl Immunohistochem Mol Morphol 2006; 14: 115–121.
Boland CR, Thibodeau SN, Hamilton SR et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: evelopment of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248–5257.
den Dunnen JT, Antonarakis SE : Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000; 15: 7–12.
Barrett JC, Fry B, Maller J, Daly MJ : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Li L, McVety S, Younan R et al. Distinct patterns of germline deletions in MLH1 and MSH2: the implication of Alu repetitive element in the genetic etiology of Lynch syndrome (HNPCC). Hum Mutat 2006; 27: 388–397.
Nystrom-Lahti M, Kristo P, Nicolaides NC et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med 1995; 1: 1203–1206.
Desai DC, Lockman JC, Chadwick RB et al. Recurrent germline mutation in MSH2 arises frequently de novo. J Med Genet 2000; 37: 646–652.
Froggatt NJ, Green J, Brassett C et al. A common MSH2 mutation in English and North American HNPCC families: origin, phenotypic expression, and sex specific differences in colorectal cancer. J Med Genet 1999; 36: 97–102.
Chan TL, Chan YW, Ho JW et al. MSH2 c.1452–1455delAATG is a founder mutation and an important cause of Hereditary Nonpolyposis Colorectal Cancer in the Southern Chinese population. Am J Hum Genet 2004; 74: 1035–1042.
Foulkes WD, Thiffault I, Gruber SB et al. The founder mutation MSH2*1906G>C is an important cause of hereditary nonpolyposis colorectal cancer in the Ashkenazi Jewish population. Am J Hum Genet 2002; 71: 1395–1412.
Lynch HT, Coronel SM, Okimoto R et al. A founder mutation of the MSH2 gene and hereditary nonpolyposis colorectal cancer in the United States. JAMA 2004; 291: 718–724.
Wagner A, Barrows A, Wijnen JT et al. Molecular analysis of hereditary nonpolyposis colorectal cancer in the United States: high mutation detection rate among clinically selected families and characterization of an American founder genomic deletion of the MSH2 gene. Am J Hum Genet 2003; 72: 1088–1100.
Chong G, Jarry J, Marcus V et al. High Frequency of Exon Deletions and Putative Founder Effects in French Canadian Lynch Syndrome Families. Hum Mutat 2009; 30: E797–E812.
Pérez-Cabornero L, Borrás Flores E, Infante Sanz M et al. Characterization of new founder Alu-mediated rearrangements in MSH2 gene associated with a Lynch syndrome phenotype. Cancer Prev Res 2011; 4: 1546–1555.
Colombino M, Cossu A, Budroni M et al. Identification of predictive factors for the occurrence of predisposing MLH1 and MSH2 germline mutations among Sardinian patients with colorectal carcinoma. Eur J Cancer 2005; 41: 1058–1064.
Hampel H, Frankel WL, Martin E et al. Screening for the Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer). N Engl J Med 2005; 352: 1851–1860.
Acknowledgements
The authors are very grateful to Prof Nicola Migone, MD, for the precious support in shaping this manuscript. We thank Mr Sergio Padovan for providing the primers for amplification of TSC2 exon 28 and Dr Patrizia Pappi for sequences purification and technical support on microsatellite analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Borelli, I., Barberis, M., Spina, F. et al. A unique MSH2 exon 8 deletion accounts for a major portion of all mismatch repair gene mutations in Lynch syndrome families of Sardinian origin. Eur J Hum Genet 21, 154–161 (2013). https://doi.org/10.1038/ejhg.2012.150
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2012.150
Keywords
This article is cited by
-
A survey of the clinicopathological and molecular characteristics of patients with suspected Lynch syndrome in Latin America
BMC Cancer (2017)
-
A founder MLH1 mutation in Lynch syndrome families from Piedmont, Italy, is associated with an increased risk of pancreatic tumours and diverse immunohistochemical patterns
Familial Cancer (2014)