Abstract
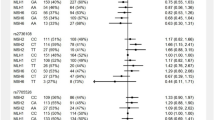
Lynch syndrome (LS) is an inherited cancer-predisposing disorder caused by germline mutations in the mismatch repair (MMR) genes. The high variability in individual cancer risk observed among LS patients suggests the existence of modifying factors. Identifying genetic modifiers of risk could help implement personalized surveillance programs based on predicted cancer risks. Here we evaluate the role of the telomerase (hTERT) rs2075786 SNP as a cancer-risk modifier in LS, studying 255 and 675 MMR gene mutation carriers from Spain and the Netherlands, respectively. The study of the Spanish sample revealed that the minor allele (A) confers increased cancer risk at an early age. The analysis of the Dutch sample confirmed the association of the A allele, especially in homozygosity, with increased cancer risk in mutation carriers under the age of 45 (relative riskLSca<45_AA=2.90; 95% confidence interval=1.02–8.26). Rs2075786 is associated with colorectal cancer (CRC) risk neither in the general population nor in non-Lynch CRC families. In silico studies predicted that the SNP causes the disruption of a transcription binding site for a retinoid receptor, retinoid X receptor alpha, probably causing early telomerase activation and therefore accelerated carcinogenesis. Notably, cancer-affected LS patients with the AA genotype have shorter telomeres than those with GG. In conclusion, MMR gene mutation carriers with hTERT rs2075786 are at high risk to develop a LS-related tumor at an early age. Cancer-preventive measures and stricter cancer surveillance at early ages might help prevent or early detect cancer in these mutation carriers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR : Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009; 76: 1–18.
Dunlop MG, Farrington SM, Carothers AD et al: Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 1997; 6: 105–110.
Aaltonen LA, Salovaara R, Kristo P et al: Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998; 338: 1481–1487.
DeFrancisco J, Grady WM : Diagnosis and management of hereditary non-polyposis colon cancer. Gastrointest Endosc 2003; 58: 390–408.
Quehenberger F, Vasen HF, van Houwelingen HC : Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet 2005; 42: 491–496.
Jenkins MA, Baglietto L, Dowty JG et al: Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol 2006; 4: 489–498.
Alarcon F, Lasset C, Carayol J et al: Estimating cancer risk in HNPCC by the GRL method. Eur J Hum Genet 2007; 15: 831–836.
Choi YH, Cotterchio M, McKeown-Eyssen G et al: Penetrance of colorectal cancer among MLH1/MSH2 carriers participating in the colorectal cancer familial registry in Ontario. Hered Cancer Clin Pract 2009; 7: 14.
Bonadona V, Bonaiti B, Olschwang S et al: Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305: 2304–2310.
Antoniou AC, Chenevix-Trench G : Common genetic variants and cancer risk in Mendelian cancer syndromes. Curr Opin Genet Dev 2010; 20: 299–307.
Scott RJ, Lubinski J : Genetic epidemiology studies in hereditary non-polyposis colorectal cancer. Methods Mol Biol 2009; 472: 89–102.
Wijnen JT, Brohet RM, van Eijk R et al: Chromosome 8q23.3 and 11q23.1 variants modify colorectal cancer risk in Lynch syndrome. Gastroenterology 2009; 136: 131–137.
Talseth-Palmer BA, Brenne IS, Ashton KA et al: Colorectal cancer susceptibility loci on chromosome 8q23.3 and 11q23.1 as modifiers for disease expression in Lynch syndrome. J Med Genet 2010; 48: 279–284.
Houlle S, Charbonnier F, Houivet E et al: Evaluation of Lynch syndrome modifier genes in 748 MMR mutation carriers. Eur J Hum Genet 2011; 19: 887–892.
Londono-Vallejo JA : Telomere length heterogeneity and chromosome instability. Cancer Lett 2004; 212: 135–144.
Meeker AK, Hicks JL, Iacobuzio-Donahue CA et al: Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res 2004; 10: 3317–3326.
Collins K, Mitchell JR : Telomerase in the human organism. Oncogene 2002; 21: 564–579.
Blasco MA, Lee HW, Hande MP et al: Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997; 91: 25–34.
Calado RT, Chen J : Telomerase: not just for the elongation of telomeres. Bioessays 2006; 28: 109–112.
McKay JD, Hung RJ, Gaborieau V et al: Lung cancer susceptibility locus at 5p15.33. Nat Genet 2008; 40: 1404–1406.
Shete S, Hosking FJ, Robertson LB et al: Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 2009; 41: 899–904.
Wrensch M, Jenkins RB, Chang JS et al: Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 2009; 41: 905–908.
Jin G, Xu L, Shu Y et al: Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis 2009; 30: 987–990.
Landi MT, Chatterjee N, Yu K et al: A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet 2009; 85: 679–691.
Rafnar T, Sulem P, Stacey SN et al: Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 2009; 41: 221–227.
Petersen GM, Amundadottir L, Fuchs CS et al: A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 2010; 42: 224–228.
Hofer P, Baierl A, Feik E et al: MNS16A tandem repeats minisatellite of human telomerase gene: a risk factor for colorectal cancer. Carcinogenesis 2011; 32: 866–871.
Hosgood HD, Cawthon R, He X, Chanock S, Lan Q : Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer 2009; 66: 157–161.
Van Dyke AL, Cote ML, Wenzlaff AS et al: Chromosome 5p Region SNPs Are Associated with Risk of NSCLC among Women. J Cancer Epidemiol 2009; 2009: 242151.
Moreno V, Gemignani F, Landi S et al: Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin Cancer Res 2006; 12: 2101–2108.
Pros E, Gomez C, Martin T, Fabregas P, Serra E, Lazaro C : Nature and mRNA effect of 282 different NF1 point mutations: focus on splicing alterations. Hum Mutat 2008; 29: E173–E193.
Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM : PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002; 18: 333–334.
Farre D, Roset R, Huerta M et al: Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res 2003; 31: 3651–3653.
Cawthon RM : Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37: e21.
Mocellin S, Verdi D, Pooley KA et al: Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst 2012; 104: 840–854.
Ding Z, Green AG, Yang X, Chernenko G, Tang SC, Pater A : Retinoic acid inhibits telomerase activity and downregulates expression but does not affect splicing of hTERT: correlation with cell growth rate inhibition in an in vitro cervical carcinogenesis/multidrug-resistance model. Exp Cell Res 2002; 272: 185–191.
Pendino F, Dudognon C, Delhommeau F et al: Retinoic acid receptor alpha and retinoid-X receptor-specific agonists synergistically target telomerase expression and induce tumor cell death. Oncogene 2003; 22: 9142–9150.
Pickett HA, Baird DM, Hoff-Olsen P et al: Telomere instability detected in sporadic colon cancers, some showing mutations in a mismatch repair gene. Oncogene 2004; 23: 3434–3443.
Mendez-Bermudez A, Hills M, Pickett HA et al: Human telomeres that contain (CTAGGG)n repeats show replication dependent instability in somatic cells and the male germline. Nucleic Acids Res 2009; 37: 6225–6238.
Rampazzo E, Bertorelle R, Serra L et al: Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer 2010; 102: 1300–1305.
Mendez-Bermudez A, Royle NJ : Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Hum Mutat 2011; 32: 939–946.
Djojosubroto MW, Choi YS, Lee HW, Rudolph KL : Telomeres and telomerase in aging, regeneration and cancer. Mol Cells 2003; 15: 164–175.
Burn J, Gerdes AM, Macrae F et al: Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378: 2081–2087.
Acknowledgements
We thank all the people responsible for genetic counseling and genetic testing in hereditary cancer at both Catalan Institute of Oncology and Leiden University Medical Center, and Gemma Aiza for technical support. This work was partly funded by the Spanish Ministry of Science and Innovation (grant BFU2009-10281 and Ramón y Cajal contract, both to LV; and fellowship to FB), the Scientific Foundation of Asociación Española Contra el Cáncer, the Carlos III Health Institute (ISCIIIRETIC: RD06/0020/1051 and RD06/0020/1050; FIS PI08/1635, FIS PI08/1359 and FIS PS09-01037; and fellowship to NS), the Catalan Health Institute and the Autonomous Government of Catalonia (2009SGR290, 2009SGR1489), and CIBERESP (CB07/02/2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Bellido, F., Guinó, E., Jagmohan-Changur, S. et al. Genetic variant in the telomerase gene modifies cancer risk in Lynch syndrome. Eur J Hum Genet 21, 511–516 (2013). https://doi.org/10.1038/ejhg.2012.204
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2012.204
Keywords
This article is cited by
-
A genetic variant in telomerase reverse transcriptase (TERT) modifies cancer risk in Lynch syndrome patients harbouring pathogenic MSH2 variants
Scientific Reports (2021)
-
Correlation of human telomerase reverse transcriptase single nucleotide polymorphisms with in vitro fertilisation outcomes
Journal of Assisted Reproduction and Genetics (2019)