Abstract
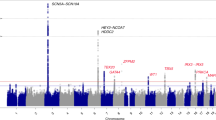
Brugada syndrome is an inherited arrhythmogenic disorder leading to sudden death predominantly in the 3–4 decade. To date the only reliable treatment is the implantation of a cardioverter defibrillator; however, better criteria for risk stratification are needed, especially for asymptomatic subjects. Brugada syndrome genetic bases have been only partially understood, accounting for <30% of patients, and have been poorly correlated with prognosis, preventing inclusion of genetic data in current guidelines. We designed an observational study to identify genetic markers for risk stratification of Brugada patients by exploratory statistical analysis. The presence of genetic variants, identified by SCN5A gene analysis and genotyping of 73 candidate polymorphisms, was correlated with the occurrence of major arrhythmic events in a cohort of 92 Brugada patients by allelic association and survival analysis. In all, 18 mutations were identified in the SCN5A gene, including 5 novel, and statistical analysis indicated that mutation carriers had a significantly increased risk of major arrhythmic events (P=0.024). In addition, we established association of five polymorphisms with major arrhythmic events occurrence and consequently elaborated a pilot risk stratification algorithm by calculating a weighted genetic risk score, including the associated polymorphisms and the presence of SCN5A mutation as function of their odds ratio. This study correlates for the first time the presence of genetic variants with increased arrhythmic risk in Brugada patients, representing a first step towards the design of a new risk stratification model.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brugada R : Sudden death: managing the family, the role of genetics. Heart 2011; 97: 676–681.
Brugada P, Brugada J : Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992; 20: 1391–1396.
Wilde AA, Antzelevitch C, Borggrefe M et al: Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation 2002; 106: 2514–2519.
Antzelevitch C, Brugada P, Borggrefe M et al: Brugada syndrome: report of the second consensus conference. Heart Rhythm 2005; 2: 429–440.
Epstein AE, DiMarco JP, Ellenbogen KA et al: ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008; 117: e350–e408.
Cummings S, Priori S : Genetics of cardiac arrhythmias. Minerva Med 2011; 102: 209–222.
Roden DM : Human genomics and its impact on arrhythmias. Trends Cardiovasc Med 2004; 14: 112–116.
Brugada R, Brugada J, Antzelevitch C et al: Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation 2000; 101: 510–515.
den Dunnen JT, Antonarakis SE : Nomenclature for the description of human sequence variations. Hum Genet 2001; 109: 121–124.
Wang Q, Li Z, Shen J, Keating MT : Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics 1996; 34: 9–16.
Pfeufer A, Sanna S, Arking DE et al: Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat Genet 2009; 41: 407–414.
Volpi S, Heaton C, Mack K et al: Whole genome association study identifies polymorphisms associated with QT prolongation during iloperidone treatment of schizophrenia. Mol Psychiatry 2009; 14: 1024–1031.
Gouas L, Nicaud V, Chaouch S et al: Confirmation of associations between ion channel gene SNPs and QTc interval duration in healthy subjects. Eur J Hum Genet 2007; 15: 974–979.
Aarnoudse AJ, Newton-Cheh C, de Bakker PI et al: Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation 2007; 116: 10–16.
Newton-Cheh C, Eijgelsheim M, Rice KM et al: Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet 2009; 41: 399–406.
Judson RS, Salisbury BA, Reed CR, Ackerman MJ : Pharmacogenetic issues in thorough QT trials. Mol Diagn Ther 2006; 10: 153–162.
Roden DM, Viswanathan PC : Genetics of acquired long QT syndrome. J Clin Invest 2005; 115: 2025–2032.
Ackerman MJ, Splawski I, Makielski JC et al: Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 2004; 1: 600–607.
Kaab S, Schulze-Bahr E : Susceptibility genes and modifiers for cardiac arrhythmias. Cardiovasc Res 2005; 67: 397–413.
Schulze-Bahr E : Arrhythmisa Predisposition. Between rare disease paradigms and common ion channel gene variants. J Am Coll Cardiol 2006; 48: 67–78.
Iwasa H, Itoh T, Nagai R, Nakamura Y, Tanaka T : Twenty single nucleotide polymorphisms (SNPs) and their allelic frequencies in four genes that are responsible for familial long QT syndrome in the Japanese population. J Hum Genet 2000; 45: 182–183.
Niu DM, Hwang B, Hwang HW et al: A common SCN5A polymorphism attenuates a severe cardiac phenotype caused by a nonsense SCN5A mutation in a Chinese family with an inherited cardiac conduction defect. J Med Genet 2006; 43: 817–821.
Poelzing S, Forleo C, Samodell M et al: SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation 2006; 114: 368–376.
Groenewegen WA, Firouzi M, Bezzina CR et al: A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res 2003; 92: 14–22.
Yang Y, Xia M, Jin Q et al: Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet 2004; 75: 899–905.
Bezzina CR, Shimizu W, Yang P et al: Common sodium channel promoter haplotype in asian subjects underlies variability in cardiac conduction. Circulation 2006; 113: 338–344.
Mango R, Vecchione L, Raso B et al: Pharmacogenomics in cardiovascular disease: the role of single nucleotide polymorphisms in improving drug therapy. Expert Opin Pharmacother 2005; 6: 2565–2576.
Weiss J, Ten Hoevel MM, Burhenne J et al: CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol 2009; 49: 196–204.
Mank-Seymour AR, Richmond JL, Wood LS et al: Association of torsades de pointes with novel and known single nucleotide polymorphisms in long QT syndrome genes. Am Heart J 2006; 152: 1116–1122.
Makita N, Mochizuki N, Tsutsui H : Absence of a trafficking defect in R1232W/T1620M, a double SCN5A mutant responsible for Brugada syndrome. Circ J 2008; 72: 1018–1019.
Anson BD, Ackerman MJ, Tester DJ et al: Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol 2004; 286: H2434–H2441.
Purcell S, Neale B, Todd-Brown K et al: PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
De Jager PL, Chibnik LB, Cui J et al: Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurol 2009; 8: 1111–1119.
Marangoni S, Di Resta C, Rocchetti M et al: A Brugada syndrome mutation (p.S216L) and its modulation by p.H558R polymorphism: standard and dynamic characterization. Cardiovasc Res 2011; 91: 606–616.
Sommariva E, Vatta M, Xi Y et al: Compound heterozygous SCN5A gene mutations in asymptomatic Brugada syndrome child. Cardiogenetics 2012; 2: 53–58.
Probst V, Allouis M, Sacher F et al: Progressive cardiac conduction defect is the prevailing phenotype in carriers of a Brugada syndrome SCN5A mutation. J Cardiovasc Electrophysiol 2006; 17: 270–275.
Meregalli PG, Tan HL, Probst V et al: Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm 2009; 6: 341–348.
Benito B, Brugada J, Brugada R, Brugada P : Brugada syndrome. Rev Esp Cardiol 2009; 62: 1297–1315.
Makimoto H, Nakagawa E, Takaki H et al: Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol 2010; 56: 1576–1584.
Nishii N, Ogawa M, Morita H et al: SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J 2010; 74: 2572–2578.
Probst V, Wilde AA, Barc J et al: SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet 2009; 2: 552–557.
Suh Y, Vijg J : SNP discovery in associating genetic variation with human disease phenotypes. Mutat Res 2005; 573: 41–53.
Splawski I, Timothy KW, Tateyama M et al: Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 2002; 297: 1333–1336.
Meadows LS, Isom LL : Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res 2005; 67: 448–458.
Wilde AA, Postema PG, Di Diego JM et al: The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol 2010; 49: 543–553.
Ackerman MJ, Mohler PJ : Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ Res 2010; 107: 457–465.
Pfister O, Oikonomopoulos A, Sereti KI et al: Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 2008; 103: 825–835.
Spooner PM : Sudden cardiac death: The larger problem... The larger genome. J CardiovascElectrophysiol 2009; 20: 585–596.
Prutkin JM, Sotoodehnia N : Genetics of sudden cardiac arrest. Prog Cardiovasc Dis 2008; 50: 390–403.
Klassen T, Davis C, Goldman A et al: Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011; 145: 1036–1048.
Acknowledgements
We are grateful to all the patients and their families. We acknowledge all personnel of the Arrhythmology Unit of San Raffaele Hospital, in particular, Francesco Sacco and Francesca Zuffada for collaboration in BrS patients’ characterization and Vincenzo Santinelli for critically revising the manuscript. We also thank Dr Amarild Cuko, Dr Ciro Indolfi, Dr Massimo Zoni Berisso, Dr Stefano Favale and Dr Luca Rocchetti for collaboration in identifying patients. We thank Alessandra Foglio and Chiara Redaelli for help in SCN5A genetic analysis and setup of SNP genotyping. This study was supported by grants from Medtronic Italy (to MF) and Italian Istituto Superiore di Sanità (ISS-526D/55 to CP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Sommariva, E., Pappone, C., Martinelli Boneschi, F. et al. Genetics can contribute to the prognosis of Brugada syndrome: a pilot model for risk stratification. Eur J Hum Genet 21, 911–917 (2013). https://doi.org/10.1038/ejhg.2012.289
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2012.289
Keywords
This article is cited by
-
Whole exome sequencing in Brugada and long QT syndromes revealed novel rare and potential pathogenic mutations related to the dysfunction of the cardiac sodium channel
Orphanet Journal of Rare Diseases (2022)
-
Pathogenesis and management of Brugada syndrome
Nature Reviews Cardiology (2016)
-
Risk Stratification and Treatment of Brugada Syndrome
Current Cardiology Reports (2014)