Abstract
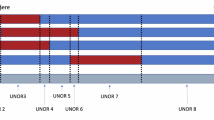
Deletions involving 17q21–q24 have been identified previously to result in two clinically recognizable contiguous gene deletion syndromes: 17q21.31 and 17q23.1–q23.2 microdeletion syndromes. Although deletions involving 17q22 have been reported in the literature, only four of the eight patients reported were identified by array-comparative genomic hybridization (array-CGH) or flourescent in situ hybridization. Here, we describe five new patients with 1.8–2.5-Mb microdeletions involving 17q22 identified by array-CGH. We also present one patient with a large karyotypically visible deletion involving 17q22, fine-mapped to ∼8.2 Mb using array-CGH. We show that the commonly deleted region in our patients spans 0.24 Mb and two genes; NOG and C17ORF67. The function of C17ORF67 is not known, whereas Noggin, the product of NOG, is essential for correct joint development. In common with the 17q22 patients reported previously, the disease phenotype of our patients includes intellectual disability, attention deficit hyperactivity disorder, conductive hearing loss, visual impairment, low set ears, facial dysmorphology and limb anomalies. All patients displayed NOG-related bone and joint features, including symphalangism and facial dysmorphology. We conclude that these common clinical features indicate a novel clinically recognizable, 17q22 contiguous microdeletion syndrome.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Dallapiccola B, Mingarelli R, Digilio C, Obregon MG, Giannotti A : Interstitial deletion del(17) (q21.3q23 or 24.2) syndrome. Clin Genet 1993; 43: 54–55.
Khalifa MM, MacLeod PM, Duncan AM : Additional case of de novo interstitial deletion del(17)(q21.3q23) and expansion of the phenotype. Clin Genet 1993; 44: 258–261.
Park JP, Moeschler JB, Berg SZ, Bauer RM, Wursterhill DH, Unique Denovo A : Interstitial Deletion Del(17) (Q21.3q23) in a Phenotypically Abnormal Infant. Clin Genet 1992; 41: 54–56.
Shimizu R, Mitsui N, Mori Y et al: Cryptic 17q22 deletion in a boy with a t(10;17)(p15.3;q22) translocation, multiple synostosis syndrome 1, and hypogonadotropic hypogonadism. Am J Med Genet A 2008; 146A: 1458–1461.
Marsh AJ, Wellesley D, Burge D et al: Interstitial deletion of chromosome 17 (del(17)(q22q23.3)) confirms a link with oesophageal atresia. J Med Genet 2000; 37: 701–704.
Khattab M, Xu F, Li P, Bhandari V : A de novo 3.54 Mb deletion of 17q22-q23.1 associated with hydrocephalus: a case report and review of literature. Am J Med Genet A 2011; 155A: 3082–3086.
Nimmakayalu M, Major H, Sheffield V et al: Microdeletion of 17q22q23.2 encompassing TBX2 and TBX4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am J Med Genet A 2011; 155A: 418–423.
Puusepp H, Zilina O, Teek R et al: 5.9 Mb microdeletion in chromosome band 17q22-q23.2 associated with tracheo-esophageal fistula and conductive hearing loss. Eur J Med Genet 2009; 52: 71–74.
Levin ML, Shaffer LG, Lewis R, Gresik MV, Lupski JR : Unique de novo interstitial deletion of chromosome 17, del(17) (q23.2q24.3) in a female newborn with multiple congenital anomalies. Am J Med Genet 1995; 55: 30–32.
Mickelson EC, Robinson WP, Hrynchak MA, Lewis ME : Novel case of del(17)(q23.1q23.3) further highlights a recognizable phenotype involving deletions of chromosome (17)(q21q24). Am J Med Genet 1997; 71: 275–279.
Thomas JA, Manchester DK, Prescott KE, Milner R, McGavran L, Cohen MM : Hunter-McAlpine craniosynostosis phenotype associated with skeletal anomalies and interstitial deletion of chromosome 17q. Am J Med Genet 1996; 62: 372–375.
Valenzuela DM, Economides AN, Rojas E et al: Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci 1995; 15: 6077–6084.
Gong Y, Krakow D, Marcelino J et al: Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet 1999; 21: 302–304.
Zimmerman LB, De Jesus-Escobar JM, Harland RM : The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 1996; 86: 599–606.
Brunet LJ, McMahon JA, McMahon AP, Harland RM : Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 1998; 280: 1455–1457.
Usami S, Abe S, Nishio S et al: Mutations in the NOG gene are commonly found in congenital stapes ankylosis with symphalangism, but not in otosclerosis. Clin Genet 2012; 82: 514–520.
Potti TA, Petty EM, Lesperance MM : A comprehensive review of reported heritable noggin-associated syndromes and proposed clinical utility of one broadly inclusive diagnostic term: NOG-related-symphalangism spectrum disorder (NOG-SSD). Hum Mutat 2011; 32: 877–886.
Ballif BC, Theisen A, McDonald-McGinn DM et al: Identification of a previously unrecognized microdeletion syndrome of 16q11.2q12.2. Clin Genet 2008; 74: 469–475.
Zhang ZF, Ruivenkamp C, Staaf J et al: Detection of submicroscopic constitutional chromosome aberrations in clinical diagnostics: a validation of the practical performance of different array platforms. Eur J Hum Genet 2008; 16: 786–792.
Traylor RN, Fan Z, Hudson B et al: Microdeletion of 6q16.1 encompassing EPHA7 in a child with mild neurological abnormalities and dysmorphic features: case report. Mol Cytogenet 2009; 2: 17.
Wikland KA, Luo ZC, Niklasson A, Karlberg J : Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr 2002; 91: 739–754.
Nellhaus G : Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics 1968; 41: 106–114.
Sharp AJ, Cheng Z, Eichler EE : Structural variation of the human genome. Annu Rev Genomics Hum Genet 2006; 7: 407–442.
Orimo A, Inoue S, Minowa O et al: Underdeveloped uterus and reduced estrogen responsiveness in mice with disruption of the estrogen-responsive finger protein gene, which is a direct target of estrogen receptor alpha. Proc Natl Acad Sci USA 1999; 96: 12027–12032.
Dawson K, Seeman P, Sebald E et al: GDF5 is a second locus for multiple-synostosis syndrome. Am J Hum Genet 2006; 78: 708–712.
Takahashi T, Takahashi I, Komatsu M et al: Mutations of the NOG gene in individuals with proximal symphalangism and multiple synostosis syndrome. Clin Genet 2001; 60: 447–451.
Brown DJ, Kim TB, Petty EM et al: Autosomal dominant stapes ankylosis with broad thumbs and toes, hyperopia, and skeletal anomalies is caused by heterozygous nonsense and frameshift mutations in NOG, the gene encoding noggin. Am J Hum Genet 2002; 71: 618–624.
Thomeer HG, Admiraal RJ, Hoefsloot L, Kunst HP, Cremers CW : Proximal symphalangism, hyperopia, conductive hearing impairment, and the NOG gene: 2 new mutations. Otol Neurotol 2011; 32: 632–638.
Weekamp HH, Kremer H, Hoefsloot LH, Kuijpers-Jagtman AM, Cruysberg JR, Cremers CW : Teunissen-Cremers syndrome: a clinical, surgical, and genetic report. Otol Neurotol 2005; 26: 38–51.
Ballif BC, Theisen A, Rosenfeld JA et al: Identification of a recurrent microdeletion at 17q23.1q23.2 flanked by segmental duplications associated with heart defects and limb abnormalities. Am J Hum Genet 2010; 86: 454–461.
Schonewolf-Greulich B, Ronan A, Ravn K et al: Two new cases with microdeletion of 17q23.2 suggest presence of a candidate gene for sensorineural hearing loss within this region. Am J Med Genet A 2011; 155A: 2964–2969.
Koolen DA, Kramer JM, Neveling K et al: Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat Genet 2012; 44: 639–641.
Koolen DA, Sharp AJ, Hurst JA et al: Clinical and molecular delineation of the 17q21.31 microdeletion syndrome. J Med Genet 2008; 45: 710–720.
Koolen DA, Vissers LE, Pfundt R et al: A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet 2006; 38: 999–1001.
Dubourg C, Sanlaville D, Doco-Fenzy M et al: Clinical and molecular characterization of 17q21.31 microdeletion syndrome in 14 French patients with mental retardation. Eur J Med Genet 2011; 54: 144–151.
Cushing H : Hereditary anchylosis of the proximal phalan-geal joints (symphalangism). Genetics 1916; 1: 90–106.
Baek GH, Lee HJ : Classification and surgical treatment of symphalangism in interphalangeal joints of the hand. Clin Orthop Surg 2012; 4: 58–65.
Acknowledgements
We thank all colleagues in pediatric departments who provided the patient samples. This work was supported by grants from the Swedish Research Council, Kronprinsessan Lovisa, Karolinska Institutet, Frimurarna Barnhuset in Stockholm and Linnea and Joseph Carlsson foundation. We would like to acknowledge Dr Regina Regan for performing the array hybridization for patient 2 in UK. SJLK is supported by the NIHR Biomedical Research Centre, Oxford with funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health. SJLK is also supported by the Wellcome Trust Core Award Grant [090532/Z/09/Z].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Laurell, T., Lundin, J., Anderlid, BM. et al. Molecular and clinical delineation of the 17q22 microdeletion phenotype. Eur J Hum Genet 21, 1085–1092 (2013). https://doi.org/10.1038/ejhg.2012.306
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2012.306
Keywords
This article is cited by
-
Aggressive Aortopathy in neonatal Marfan syndrome
Journal of Congenital Cardiology (2019)