Abstract
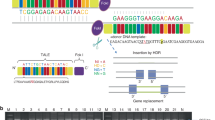
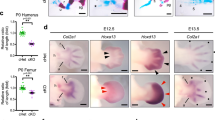
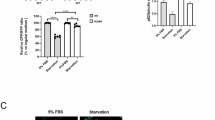
Acromesomelic chondrodysplasias (ACDs) are characterized by disproportionate shortening of the appendicular skeleton, predominantly affecting the middle (forearms and forelegs) and distal segments (hands and feet). Here, we present two consanguineous families with missense (c.157T>C, p.(C53R)) or nonsense (c.657G>A, p.(W219*)) mutations in BMPR1B. Homozygous affected individuals show clinical and radiographic findings consistent with ACD-type Grebe. Functional analysis of the missense mutation C53R revealed that the mutated receptor was partially located at the cell membrane. In contrast to the wild-type receptor, C53R mutation hindered the activation of the receptor by its ligand GDF5, as shown by reporter gene assay. Further, overexpression of the C53R mutation in an in vitro chondrogenesis assay showed no effect on cell differentiation, indicating a loss of function. The nonsense mutation (c.657G>A, p.(W219*)) introduces a premature stop codon, which is predicted to be subject to nonsense-mediated mRNA decay, causing reduced protein translation of the mutant allele. A loss-of-function effect of both mutations causing recessive ACD-type Grebe is further supported by the mild brachydactyly or even non-penetrance of these mutations observed in the heterozygous parents. In contrast, dominant-negative BMPR1B mutations described previously are associated with autosomal-dominant brachydactyly-type A2.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kornak U, Mundlos S : Genetic disorders of the skeleton: a developmental approach. Am J Hum Genet 2003; 73: 447–474.
Warman ML, Cormier-Daire V, Hall C et al: Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet Part A 2011; 155A: 943–968.
Nickel J, Kotzsch A, Sebald W, Mueller TD : A single residue of GDF-5 defines binding specificity to BMP receptor IB. J Mol Biol 2005; 349: 933–947.
Al-Yahyaee SA, Al-Kindi MN, Habbal O, Kumar DS : Clinical and molecular analysis of Grebe acromesomelic dysplasia in an Omani family. Am J Med Genet Part A 2003; 121A: 9–14.
Basit S, Naqvi SK, Wasif N, Ali G, Ansar M, Ahmad W : A novel insertion mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene underlies Grebe-type chondrodysplasia in a consanguineous Pakistani family. BMC Med Genet 2008; 9: 102.
Douzgou S, Lehmann K, Mingarelli R, Mundlos S, Dallapiccola B : Compound heterozygosity for GDF5 in Du Pan type chondrodysplasia. Am J Med Genet Part A 2008; 146A: 2116–2121.
Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH et al: Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome). Clin Genet 2002; 61: 454–458.
Faiyaz-Ul-Haque M, Faqeih EA, Al-Zaidan H, Al-Shammary A, Zaidi SH : Grebe-type chondrodysplasia: a novel missense mutation in a conserved cysteine of the growth differentiation factor 5. J Bone Miner Metab 2008; 26: 648–652.
Savarirayan R, White SM, Goodman FR et al: Broad phenotypic spectrum caused by an identical heterozygous CDMP-1 mutation in three unrelated families. Am J Med Genet Part A 2003; 117A: 136–142.
Stelzer C, Winterpacht A, Spranger J, Zabel B : Grebe dysplasia and the spectrum of CDMP1 mutations. Pediatr Pathol Molec Med 2003; 22: 77–85.
Szczaluba K, Hilbert K, Obersztyn E, Zabel B, Mazurczak T, Kozlowski K : Du Pan syndrome phenotype caused by heterozygous pathogenic mutations in CDMP1 gene. Am J Med Genet Part A 2005; 138: 379–383.
Thomas JT, Kilpatrick MW, Lin K et al: Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet 1997; 17: 58–64.
Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP : A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat Genet 1996; 12: 315–317.
Demirhan O, Turkmen S, Schwabe GC et al: A homozygous BMPR1B mutation causes a new subtype of acromesomelic chondrodysplasia with genital anomalies. J Med Genet 2005; 42: 314–317.
Lehmann K, Seemann P, Stricker S et al: Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci USA 2003; 100: 12277–12282.
Curtis D : Heterozygote expression in Grebe chondrodysplasia. Clin Genet 1986; 29: 455–456.
Holder-Espinasse M, Escande F, Mayrargue E et al: Angel shaped phalangeal dysplasia, hip dysplasia, and positional teeth abnormalities are part of the brachydactyly C spectrum associated with CDMP-1 mutations. J Med Genet 2004; 41: e78.
Everman DB, Bartels CF, Yang Y et al: The mutational spectrum of brachydactyly type C. Am J Med Genet 2002; 112: 291–296.
Gutierrez-Amavizca BE, Brambila-Tapia AJ, Juarez-Vazquez CI et al: A novel mutation in CDMP1 causes brachydactyly type C with "angel-shaped phalanx". A genotype-phenotype correlation in the mutational spectrum. Eur J Med Genet 2012; 55: 611–614.
Polinkovsky A, Robin NH, Thomas JT et al: Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat Genet 1997; 17: 18–19.
Lehmann K, Seemann P, Boergermann J et al: A novel R486Q mutation in BMPR1B resulting in either a brachydactyly type C/symphalangism-like phenotype or brachydactyly type A2. Eur J Hum Genet 2006; 14: 1248–1254.
Seemann P, Schwappacher R, Kjaer KW et al: Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J Clin Invest 2005; 115: 2373–2381.
Schwabe GC, Turkmen S, Leschik G et al: Brachydactyly type C caused by a homozygous missense mutation in the prodomain of CDMP1. Am J Med Genet Part A 2004; 124A: 356–363.
Goujon M, McWilliam H, Li W et al: A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res 2010; 38: W695–W699.
Sievers F, Wilm A, Dineen D et al: Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7: 539.
Goodstadt L, Ponting CP : CHROMA: consensus-based colouring of multiple alignments for publication. Bioinformatics 2001; 17: 845–846.
Kotzsch A, Nickel J, Seher A, Sebald W, Muller TD : Crystal structure analysis reveals a spring-loaded latch as molecular mechanism for GDF-5-type I receptor specificity. EMBO J 2009; 28: 937–947.
Logan M, Tabin C : Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses. Methods 1998; 14: 407–420.
Korchynskyi O, ten Dijke P : Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 2002; 277: 4883–4891.
Hampf M, Gossen M : A protocol for combined Photinus and Renilla luciferase quantification compatible with protein assays. Anal Biochem 2006; 356: 94–99.
Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P : Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol 1987; 61: 3004–3012.
Stricker S, Fundele R, Vortkamp A, Mundlos S : Role of Runx genes in chondrocyte differentiation. Dev Biol 2002; 245: 95–108.
Hamburger V, Hamilton HL : A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 1992; 195: 231–272.
Kervestin S, Jacobson A : NMD: a multifaceted response to premature translational termination. Nat Rev Mol Cell Biol 2012; 13: 700–712.
DeLise AM, Stringa E, Woodward WA, Mello MA, Tuan RS : Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol 2000; 137: 359–375.
Ellgaard L, Helenius A : Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 2003; 4: 181–191.
Robinson PN, Kohler S, Bauer S, Seelow D, Horn D, Mundlos S : The Human Phenotype Ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet 2008; 83: 610–615.
Acknowledgements
We thank the family members who participated in this study. Contributions were made possible by DFG funding through the Berlin-Brandenburg School for Regenerative Therapies GSC 203 (UW, AD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Graul-Neumann, L., Deichsel, A., Wille, U. et al. Homozygous missense and nonsense mutations in BMPR1B cause acromesomelic chondrodysplasia-type Grebe. Eur J Hum Genet 22, 726–733 (2014). https://doi.org/10.1038/ejhg.2013.222
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2013.222
Keywords
This article is cited by
-
Linked homozygous BMPR1B and PDHA2 variants in a consanguineous family with complex digit malformation and male infertility
European Journal of Human Genetics (2018)
-
BMP signalling in skeletal development, disease and repair
Nature Reviews Endocrinology (2016)
-
A hypomorphic BMPR1B mutation causes du Pan acromesomelic dysplasia
Orphanet Journal of Rare Diseases (2015)
-
Brachydactyly Type C patient with compound heterozygosity for p.Gly319Val and p.Ile358Thr variants in the GDF5 proregion: benign variants or mutations?
Journal of Human Genetics (2015)
-
Two novel disease-causing variants in BMPR1B are associated with brachydactyly type A1
European Journal of Human Genetics (2015)