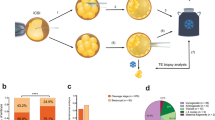
Abstract
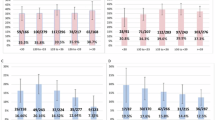
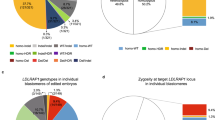
Our study provides an analysis of the outcome of meiotic segregation of three-way translocations in cleavage-stage embryos and the accuracy and limitations of preimplantation genetic diagnosis (PGD) using the fluorescence in situ hybridization technique. We propose a general model for estimating reproductive risks for carriers of this class of complex chromosome rearrangement. The data presented describe six cycles for four couples where one partner has a three-way translocation. For male heterozygotes, 27.6% of embryos were consistent with 3:3 alternate segregation resulting in a normal or balanced translocation chromosome complement; 41.4% were consistent with 3:3 adjacent segregation of the translocations, comprising 6.9% reflecting adjacent-1 and 34.5% adjacent-2 segregation; 24.1% were consistent with 4:2 nondisjunction; none showed 5:1 or 6:0 segregation; the probable mode could not be ascertained for 6.9% of embryos due to complex mosaicism or nucleus fragmentation. The test accuracy for male heterozygotes was estimated to be 93.1% with 100% sensitivity and 75% specificity. With 72.4% prevalence, the predictive value was estimated to be 91.3% for an abnormal test result and 100% for a normal test result. Two of four couples had a healthy baby following PGD. The proportion of normal/balanced embryo could be significantly less for female heterozygotes, and our model indicates that this could be detrimental to the effectiveness of PGD. A 20% risk of live-born offspring with an unbalanced translocation is generally accepted, largely based on the obstetric history of female heterozygotes; we suggest that a 3% risk may be more appropriate for male carriers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Madan K : What is a complex chromosome rearrangement? Am J Med Genet Part A 2013; 161A: 1181–1184.
Pai GS, Thomas GH, Mahoney W, Migeon BR : Complex chromosome rearrangements. Report of a new case and literature review. Clin Genet 1980; 18: 436–444.
Kausch K, Haaf T, Köhler J, Schmid M : Complex chromosomal rearrangement in a woman with multiple miscarriages. Am J Med Genet 1988; 31: 415–420.
Madan K, Nieuwint AW, van Bever Y : Recombination in a balanced complex translocation of a mother leading to a balanced reciprocal translocation in the child. Review of 60 cases of balanced complex translocations. Hum Genet 1997; 99: 806–815.
Madan K : Balanced complex chromosome rearrangements: reproductive aspects. A review. Am J Med Genet A 2012; 158A: 947–963.
Pellestor F, Anahory T, Lefort G et al: Complex chromosomal rearrangements: origin and meiotic behavior. Hum Reprod Update 2011a; 17: 476–494.
De Gregori M, Ciccone R, Magini P et al: Cryptic deletions are a common finding in "balanced" reciprocal and complex chromosome rearrangements: a study of 59 patients. J Med Genet 2007; 44: 750–762.
Feenstra I, Hanemaaijer N, Sikkema-Raddatz B et al: Balanced into array: genome-wide array analysis in 54 patients with an apparently balanced de novo chromosome rearrangement and a meta-analysis. Eur J Hum Genet 2011; 19: 1152–1160.
Ogilvie CM, Raymond FL, Harrison RH, Scriven PN, Docherty Z : A new approach to the elucidation of complex chromosome rearrangements illustrated by a case of Rieger syndrome. J Med Genet 1998; 35: 234–237.
Saadallah N, Hulten M : A complex three breakpoint translocation involving chromosomes 2, 4, and 9 identified by meiotic investigations of a human male ascertained for subfertility. Hum Genet 1985; 71: 312–320.
Johannisson R, Löhrs U, Passarge E : Pachytene analysis in males heterozygous for a familial translocation (9;12;13) (q22; q22; q32) ascertained through a child with partial trisomy 9. Cytogenet Cell Genet 1988; 47: 160–166.
Pickering S, Polidoropoulos N, Caller J, Scriven P, Ogilvie CM, Braude P, Preimplantation Genetic Diagnosis Study Group: Strategies and outcomes of the first 100 cycles of preimplantation genetic diagnosis at the Guy's and St. Thomas' Center. Fertil Steril 2003; 79: 81–90.
Scriven PN, Kirby TL, Ogilvie CM : FISH for pre-implantation genetic diagnosis. J Vis Exp 2011; 48: e2570.
Scriven PN, Handyside AH, Ogilvie CM : Chromosome translocations: segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn 1998; 18: 1437–1449.
Mackie OC, Scriven PN : Meiotic outcomes in reciprocal translocation carriers ascertained in 3-day human embryos. Eur J Hum Genet 2002; 10: 801–806.
Scriven PN, Flinter FA, Khalaf Y, Lashwood A, Mackie OC : Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur J Hum Genet 2013; 21: 1035–1041.
Bint SM, Ogilvie CM, Flinter FA, Khalaf Y, Scriven PN : Meiotic segregation of Robertsonian translocations ascertained in cleavage-stage embryos—implications for preimplantation genetic diagnosis. Hum Reprod 2011; 26: 1575–1584.
Pellestor F, Puechberty J, Weise A et al: Meiotic segregation of complex reciprocal translocations: direct analysis of the spermatozoa of a t(5;13;14) carrier. Fertil Steril 2011b; 95: e17–e22.
ISCN (2013): An International System for Human Cytogenetic Nomenclature, Shaffer LG, McGowan-Jordan J, Schmid M (eds), S. Karger: Basel, Switzerland, 2013.
Stengel-Rutkowski S, Stene J, Gallano P : Risk estimates in balanced parental reciprocal translocations. Monographies des Annales de Génétique 1988, Expansion Scientifique Française, Paris.
Gardner RJM, Sutherland GR, Shaffer LG : Chromosome Abnormalities and Genetic Counseling 4th Ed 2012 Oxford University Press: New York, USA.
Scriven PN, Bossuyt PM : Diagnostic accuracy: theoretical models for preimplantation genetic testing of a single nucleus using the fluorescence in situ hybridization technique. Hum Reprod 2010; 25: 2622–2628.
Cifuentes P, Navarro J, Míguez L, Egozcue J, Benet J : Sperm segregation analysis of a complex chromosome rearrangement, 2;22;11, by whole chromosome painting. Cytogenet Cell Genet 1998; 82: 204–209.
Loup V, Bernicot I, Janssens P et al: Combined FISH and PRINS sperm analysis of complex chromosome rearrangement t(1;19;13): an approach facilitating PGD. Mol Hum Reprod 2010; 16: 111–116.
Estop AM, Van Kirk V, Cieply K : Segregation analysis of four translocations, t(2;18), t(3;15), t(5;7), and t(10;12), by sperm chromosome studies and a review of the literature. Cytogenet Cell Genet 1995; 70: 80–87.
Escudero T, Estop A, Fischer J, Munne S : Preimplantation genetic diagnosis for complex chromosome rearrangements. Am J Med Genet A 2008; 146A: 1662–1669.
Lim CK, Cho JW, Kim JY, Kang IS, Shim SH, Jun JH : A healthy live birth after successful preimplantation genetic diagnosis for carriers of complex chromosome rearrangements. Fertil Steril 2008; 90: 1680–1684.
Munné S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J : Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril 2000; 73: 1209–1218.
Gorski JL, Kistenmacher ML, Punnett HH, Zackai EH, Emanuel BS : Reproductive risks for carriers of complex chromosome rearrangements: analysis of 25 families. Am J Med Genet 1988; 29: 247–261.
Vanneste E, Melotte C, Voet T et al: PGD for a complex chromosomal rearrangement by array comparative genomic hybridization. Hum Reprod 2011; 26: 941–949.
Alfarawati S, Fragouli E, Colls P, Wells D : First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Hum Reprod 2011; 26: 1560–1574.
Fiorentino F, Spizzichino L, Bono S et al: PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod 2011; 26: 1925–1935.
Treff NR, Northrop LE, Kasabwala K, Su J, Levy B, Scott RT Jr. : Single nucleotide polymorphism microarray-based concurrent screening of 24-chromosome aneuploidy and unbalanced translocations in preimplantation human embryos. Fertil Steril 2011; 95: 1606–1612.
van Uum CM, Stevens SJ, Dreesen JC et al: SNP array-based copy number and genotype analyses for preimplantation genetic diagnosis of human unbalanced translocations. Eur J Hum Genet 2012; 20: 938–944.
Stene J, Stengel-Rutkowski S : Genetic risks of familial reciprocal and Robertsonian translocation carriers; In Daniel A (ed.): The Cytogenetics of mammalian autosomal rearrangements. Alan R Liss: New York, USA, 1988, pp 3–72.
Acknowledgements
We are grateful to Toby Kirby for his first-class technical expertise.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Scriven, P., Bint, S., Davies, A. et al. Meiotic outcomes of three-way translocations ascertained in cleavage-stage embryos: refinement of reproductive risks and implications for PGD. Eur J Hum Genet 22, 748–753 (2014). https://doi.org/10.1038/ejhg.2013.237
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2013.237
Keywords
This article is cited by
-
Characterization of a complex chromosomal rearrangement involving chromosomes 1, 3, and 4 in a slightly affected male with bad obstetrics history
Journal of Assisted Reproduction and Genetics (2018)
-
A novel male 2;4;14 complex chromosomal translocation with normal semen parameters but 100% embryonic aneuploidy
Journal of Assisted Reproduction and Genetics (2018)