Abstract
So far, the role of mutations in the δ-sarcogylcan (Sgcd) gene in causing autosomal dominant dilated cardiomyopathy (DCM) remains inconclusive. A p.S151A missense mutation in exon 6 of the Sgcd gene was reported to cause severe isolated autosomal dominant DCM without affecting skeletal muscle. This is controversial to our previous findings in a large consanguineous family where this p.S151A mutation showed no relevance for cardiac disease. In this study, the potential of the p.S151A mutation to cause DCM was investigated by using two different approaches: (1) engineering and characterization of heterozygous knock-in (S151A-) mice carrying the p.S151A mutation and (2) evaluation of the potential of adeno-associated virus (AAV) 9-based cardiac-specific transfer of p.S151A-mutated Sgcd cDNA to rescue the cardiac phenotype in Sgcd-deficient (Sgcd-null) mice as it has been demonstrated for intact, wild-type Sgcd cDNA. Heterozygous S151A knock-in mice developed a rather mild phenotype of cardiomyopathy. Increased heart to body weight suggests cardiac enlargement in 1-year-old S151A knock-in mice. However, at this age cardiac function, assessed by echocardiography, is maintained and histopathology completely absent. Myocardial expression of p.S151A cDNA, similar to intact Sgcd cDNA, restores cardiac function, although not being able to prevent myocardial histopathology in Sgcd-null mice completely. Our results suggest that the p.S151A mutation causes a mild, subclinical phenotype of cardiomyopathy, which is prone to be overseen in patients carrying such sequence variants. Furthermore, this study shows the suitability of an AAV-mediated cardiac gene transfer approach to analyze whether a sequence variant is a disease-causing mutation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Heydemann A, McNally EM : Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med 2007; 17: 55–59, Review.
Lapidos KA, Kakkar R, McNally EM : The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res 2004; 94: 1023–1031.
Yoshida T, Pan Y, Hanada H, Iwata Y, Shigekawan M : Bidirectional signaling between sarcoglycans and the integrin adhesion system in cultured L6 myocytes. J Biol Chem 1998; 273: 1583–1590.
Yoshida M, Hama H, Ishikawa-Sakurai M et al: Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet 2000; 9: 1033–1040.
Zatz M, de Paula F, Starling A, Vainzof M : The 10 autosomal recessive limb-girdle muscular dystrophies. Neuromuscul Disord 2003; 13: 532–544.
Sandonà D, Betto R : Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert Rev Mol Med 2009; 11: e28.
Politano L, Nigro V, Passamano L et al: Evaluation of cardiac and respiratory involvement in sarcoglycanopathies. Neuromuscul Disord 2001; 11: 178–185.
Hack AA, Lam MY, Cordier L et al: Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J Cell Sci 2000; 113: 2535–2544.
Coral-Vazquez R, Cohn RD, Moore SA et al: Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell 1999; 98: 465–474.
Tsubata S, Bowles KR, Vatta M et al: Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest 2000; 106: 655–662.
Kärkkäinen S, Miettinen R, Tuomainen P et al: A novel mutation, Arg71Thr, in the delta-sarcoglycan gene is associated with dilated cardiomyopathy. J Mol Med 2003; 81: 795–800.
Heydemann A, Demonbreun A, Hadhazy M, Earley JU, McNally EM : Nuclear sequestration of delta-sarcoglycan disrupts the nuclear localization of lamin A/C and emerin in cardiomyocytes. Hum Mol Genet 2007; 16: 355–363.
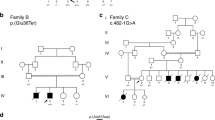
Bauer R, Hudson J, Müller HD et al: Does δ-sarcoglycan-associated autosomal dominant cardiomyopathy exist? Eur J Hum Genet 2009; 17: 1148–1153.
Goehringer C, Rutschow D, Bauer R et al: Prevention of cardiomyopathy in δ-sarcoglycan knock-out mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res 2009; 82: 404–410.
Zang P, Li MZ, Elledge SJ : Towards genetic genomeprojects: genomic library screening and gene-targeting vector construction in a single step. Nat Genet 2002; 30: 31–39.
Liu P, Jenkins NA, Copeland NG : A highly efficient recombineering-based method for generating conditional knockout mutations. Genome 2003; 13: 476–484.
Geiger SK, Bär H, Ehlermann P et al: Incomplete nonsense-mediated decay of mutant lamin A/C mRNA provokes dilated cardiomyopathy and ventricular tachycardia. J Mol Med 2008; 86: 281–289.
Müller OJ, Schinkel S, Kleinschmidt JA, Katus HA, Bekeredjian R : Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther 2008; 15: 1558–1565.
Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N : Production and purification of recombinant adeno-associated virus. Methods Enzymol 2000; 316: 743–761.
Jung D, Duclos F, Apostol B : Characterization of delta-sarcoglycan, a novel component of the oligomeric sarcoglycan complex involved in limb-girdle muscular dystrophy. J Biol Chem 1996; 271: 32321–32329.
Morimoto S : Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res 2008; 77: 659–666.
Zhu X, Hadhazy M, Groh ME, Wheeler MT, Wollmann R, McNally EM : Overexpression of gamma-sarcoglycan induces severe muscular dystrophy. Implications for the regulation of Sarcoglycan assembly. J Biol Chem 2001; 276: 21785–21790.
Haruyama N, Cho A, Kulkarni AB : Overview: engineering transgenic constructs and mice. Curr Protoc Cell Biol 2009;, Unit 19.10, doi:10.1002/0471143030.cb1910s42.
Danialou G, Comtois AS, Dudley R et al: Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J 2001; 15: 1655–1657.
Müller OJ, Leuchs B, Pleger ST : Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors. Cardiovasc Res 2006; 70: 70–78.
Müller OJ, Katus HA, Bekeredjian R : Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc Res 2007; 73: 453–462.
Inagaki K, Fuess S, Storm TA et al: Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006; 14: 45–53.
Pacak CA, Mah CS, Thattaliyath BD et al: Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006; 99: e3–e9.
Gouveia TL, Kossugue PM, Paim JF et al: A new evidence for the maintenance of the sarcoglycan complex in muscle sarcolemma in spite of the primary absence of d-SG protein. J Mol Med 2007; 85: 415–420.
Acknowledgements
We thank Barbara Leuchs and the DKFZ vector core production unit for generating high titer AAV vector stocks. In addition, we thank Ulrike Gärtner (University of Heidelberg) for skillful intravenous injections of AAV vectors and Ursel Warncke (Max-Planck Institute for Medical Research, Heidelberg) for homologous recombination of ES cells. We further thank Stefan Selbert, PhD, (Polygene AG, Rümlang, Switzerland) for help in analyzing homologous recombination and the Nikon Center Heidelberg for support in microscopy. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (MU 1654/3-2 to OJM); and the DZHK (German Centre for Cardiovascular Research) and the BMBF (German Ministry of Education and Research) to OJM and HAK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Rutschow, D., Bauer, R., Göhringer, C. et al. S151A δ-sarcoglycan mutation causes a mild phenotype of cardiomyopathy in mice. Eur J Hum Genet 22, 119–125 (2014). https://doi.org/10.1038/ejhg.2013.97
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2013.97
Keywords
This article is cited by
-
The role of the dystrophin glycoprotein complex in muscle cell mechanotransduction
Communications Biology (2022)