Abstract
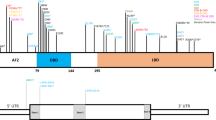
Noonan syndrome (NS) is a developmental disorder characterized by short stature, facial dysmorphisms and congenital heart defects. To date, all mutations known to cause NS are dominant, activating mutations in signal transducers of the RAS/mitogen-activated protein kinase (MAPK) pathway. In 25% of cases, however, the genetic cause of NS remains elusive, suggesting that factors other than those involved in the canonical RAS/MAPK pathway may also have a role. Here, we used family-based whole exome sequencing of a case–parent trio and identified a de novo mutation, p.(Arg802His), in A2ML1, which encodes the secreted protease inhibitor α-2-macroglobulin (A2M)-like-1. Subsequent resequencing of A2ML1 in 155 cases with a clinical diagnosis of NS led to the identification of additional mutations in two families, p.(Arg802Leu) and p.(Arg592Leu). Functional characterization of these human A2ML1 mutations in zebrafish showed NS-like developmental defects, including a broad head, blunted face and cardiac malformations. Using the crystal structure of A2M, which is highly homologous to A2ML1, we identified the intramolecular interaction partner of p.Arg802. Mutation of this residue, p.Glu906, induced similar developmental defects in zebrafish, strengthening our conclusion that mutations in A2ML1 cause a disorder clinically related to NS. This is the first report of the involvement of an extracellular factor in a disorder clinically related to RASopathies, providing potential new leads for better understanding of the molecular basis of this family of developmental diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Mendez HM, Opitz JM : Noonan syndrome: a review. Am J Med Genet 1985; 21: 493–506.
Allanson JE : Noonan syndrome. J Med Genet 1987; 24: 9–13.
van der Burgt I : Noonan syndrome. Orphanet J Rare Dis 2007; 2: 4.
Roberts AE, Allanson JE, Tartaglia M, Gelb BD : Noonan syndrome. Lancet 2013; 381: 333–342.
Colquitt JL, Noonan JA : Cardiac findings in Noonan syndrome on long-term follow-up. Congen Heart Dis 2013; 9: 144–150.
Aoki Y, Niihori T, Banjo T et al: Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet 2013; 93: 173–180.
Tartaglia M, Gelb BD, Zenker M : Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab 2011; 25: 161–179.
de Ligt J, Willemsen MH, van Bon BW et al: Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 2012; 367: 1921–1929.
Bell JB, D., Sistermans E, Ramsden SC Practice guidelines for the Interpretation and Reporting of Unclassified Variants (UVs) in Clinical Molecular Genetics, 2007 Available at: http://www.cmgs.org/bpgs/pdfs%20current%20bpgs/UV%20 GUIDELINES%20ratified.pdf (last accessed 2 June 2014).
Krieger E, Vriend G : Models@Home: distributed computing in bioinformatics using a screensaver based approach. Bioinformatics 2002; 18: 315–318.
Venselaar H, Te Beek TA, Kuipers RK, Hekkelman ML, Vriend G : Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform 2010; 11: 548.
Jopling C, van Geemen D, den Hertog J : Shp2 knockdown and Noonan/LEOPARD mutant Shp2-induced gastrulation defects. PLoS Genet 2007; 3: e225.
Thisse C, Thisse B : High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 2008; 3: 59–69.
Yelon D, Horne SA, Stainier DY : Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 1999; 214: 23–37.
Roach JC, Glusman G, Smit AF et al: Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 2010; 328: 636–639.
Marrero A, Duquerroy S, Trapani S et al: The crystal structure of human alpha2-macroglobulin reveals a unique molecular cage. Angew Chem 2012; 51: 3340–3344.
Hong SK, Dawid IB : Alpha2 macroglobulin-like is essential for liver development in zebrafish. PLoS One 2008; 3: e3736.
Runtuwene V, van Eekelen M, Overvoorde J et al: Noonan syndrome gain-of-function mutations in NRAS cause zebrafish gastrulation defects. Dis Models Mech 2011; 4: 393–399.
Galliano MF, Toulza E, Gallinaro H et al: A novel protease inhibitor of the alpha2-macroglobulin family expressed in the human epidermis. J Biol Chem 2006; 281: 5780–5789.
Galliano MF, Toulza E, Jonca N, Gonias SL, Serre G, Guerrin M : Binding of alpha2ML1 to the low density lipoprotein receptor-related protein 1 (LRP1) reveals a new role for LRP1 in the human epidermis. PLoS One 2008; 3: e2729.
Geetha N, Mihaly J, Stockenhuber A et al: Signal integration and coincidence detection in the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) cascade: concomitant activation of receptor tyrosine kinases and of LRP-1 leads to sustained ERK phosphorylation via down-regulation of dual specificity phosphatases (DUSP1 and -6). J Biol Chem 2011; 286: 25663–25674.
Takayama Y, May P, Anderson RG, Herz J : Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor beta (PDGFR beta). J Biol Chem 2005; 280: 18504–18510.
Tartaglia M, Kalidas K, Shaw A et al: PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype–phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet 2002; 70: 1555–1563.
Cordeddu V, Di Schiavi E, Pennacchio LA et al: Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet 2009; 41: 1022–1026.
Zenker M, Voss E, Reis A : Mild variable Noonan syndrome in a family with a novel PTPN11 mutation. Eur J Med Genet 2007; 50: 43–47.
Oishi K, Gaengel K, Krishnamoorthy S et al: Transgenic Drosophila models of Noonan syndrome causing PTPN11 gain-of-function mutations. Hum Mol Genet 2006; 15: 543–553.
Martinelli S, De Luca A, Stellacci E et al: Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet 2010; 87: 250–257.
Acknowledgements
We thank Hanka Venselaar for bioinformatics support in protein modeling and Martina Ruiterkamp-Versteeg, Petra de Vries, Suzanne Keijzers-Vloet and Martine van Zweeden for technical assistance. This work was funded, in part, by a grant from the Research Council for Earth and Life Sciences (ALW 819.02.021) with financial aid from the Netherlands Organization for Scientific Research (NWO) (to JdH), Telethon-Italy (GGP13107 to MT), Fundação para a Ciência e Tecnologia (PTDC/BIM-MEC/0650/2012 to JLC) and PPS5 Consórcio DoIT (ADI – Agência de Inovação to JLC). IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Education and Science and is partially supported by FCT, the Portuguese Foundation for Science and Technology.
Deposition of genetic data
The data obtained in this study are submitted to LOVD, an online gene-centered collection and display of DNA variations (http://databases.lovd.nl/shared/genes).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Vissers, L., Bonetti, M., Paardekooper Overman, J. et al. Heterozygous germline mutations in A2ML1 are associated with a disorder clinically related to Noonan syndrome. Eur J Hum Genet 23, 317–324 (2015). https://doi.org/10.1038/ejhg.2014.115
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2014.115
This article is cited by
-
Molecular and clinical profile of patients referred as Noonan or Noonan-like syndrome in Greece: a cohort of 86 patients
European Journal of Pediatrics (2022)
-
Integrated in silico MS-based phosphoproteomics and network enrichment analysis of RASopathy proteins
Orphanet Journal of Rare Diseases (2021)
-
The clinical significance of A2ML1 variants in Noonan syndrome has to be reconsidered
European Journal of Human Genetics (2021)
-
LZTR1 facilitates polyubiquitination and degradation of RAS-GTPases
Cell Death & Differentiation (2020)
-
Delineation of LZTR1 mutation-positive patients with Noonan syndrome and identification of LZTR1 binding to RAF1–PPP1CB complexes
Human Genetics (2019)