Abstract
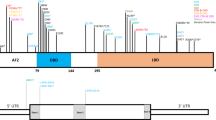
De novo monoallelic variants in NFIX cause two distinct syndromes. Whole gene deletions, nonsense variants and missense variants affecting the DNA-binding domain have been seen in association with a Sotos-like phenotype that we propose is referred to as Malan syndrome. Frameshift and splice-site variants thought to avoid nonsense-mediated RNA decay have been seen in Marshall–Smith syndrome. We report six additional patients with Malan syndrome and de novo NFIX deletions or sequence variants and review the 20 patients now reported. The phenotype is characterised by moderate postnatal overgrowth and macrocephaly. Median height and head circumference in childhood are 2.0 and 2.3 standard deviations (SD) above the mean, respectively. There is overlap of the facial phenotype with NSD1-positive Sotos syndrome in some cases including a prominent forehead, high anterior hairline, downslanting palpebral fissures and prominent chin. Neonatal feeding difficulties and/or hypotonia have been reported in 30% of patients. Developmental delay/learning disability have been reported in all cases and are typically moderate. Ocular phenotypes are common, including strabismus (65%), nystagmus (25% ) and optic disc pallor/hypoplasia (25%). Other recurrent features include pectus excavatum (40%) and scoliosis (25%). Eight reported patients have a deletion also encompassing CACNA1A, haploinsufficiency of which causes episodic ataxia type 2 or familial hemiplegic migraine. One previous case had episodic ataxia and one case we report has had cyclical vomiting responsive to pizotifen. In individuals with this contiguous gene deletion syndrome, awareness of possible later neurological manifestations is important, although their penetrance is not yet clear.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Neylon OM, Werther GA, Sabin MA : Overgrowth syndromes. Curr Opin Pediatr 2012; 24: 505–511.
Douglas J, Hanks S, Temple IK et al: NSD1 mutations are the major cause of Sotos syndrome and occur in some cases of Weaver syndrome but are rare in other overgrowth phenotypes. Am J Hum Genet 2003; 72: 132–143.
De Boer L, Van Duyvenvoorde HA, Willemstein-Van Hove EC et al: Mutations in the NSD1 gene in patients with Sotos syndrome associate with endocrine and paracrine alterations in the IGF system. Eur J Endocrinol 2004; 151: 333–341.
Auvin S, Holder-Espinasse M, Lamblin MD, Andrieux J : Array-CGH detection of a de novo 0.7-Mb deletion in 19p13.13 including CACNA1A associated with mental retardation and epilepsy with infantile spasms. Epilepsia 2009; 50: 2501–2503.
Dolan M, Mendelsohn NJ, Pierpont ME, Schimmenti LA, Berry SA, Hirsch B : A novel microdeletion/microduplication syndrome of 19p13.13. Genet Med 2010; 12: 503–511.
Yoneda Y, Saitsu H, Touyama M et al: Missense mutations in the DNA-binding/dimerization domain of NFIX cause Sotos-like features. J Hum Genet 2012; 57: 207–211.
Malan V, Rajan D, Thomas S et al: Distinct effects of allelic NFIX mutations on nonsense-mediated mRNA decay engender either a Sotos-like or a Marshall-Smith syndrome. Am J Hum Genet 2010; 87: 189–198.
Priolo M, Grosso E, Mammi C et al: A peculiar mutation in the DNA-binding/dimerization domain of NFIX causes Sotos-like overgrowth syndrome: a new case. Gene 2012; 511: 103–105.
Nimmakayalu M, Horton VK, Darbro B et al: Apparent germline mosaicism for a novel 19p13.13 deletion disrupting NFIX and CACNA1A. Am J Med Genet A 2013; 161: 1105–1109.
Lysy PA, Ravoet M, Wustefeld S et al: A new case of syndromic craniosynostosis with cryptic 19p13.2-p13.13 deletion. Am J Med Genet A 2009; 149A: 2564–2568.
Lehman AM, du Souich C, Chai D et al: 19p13.2 microduplication causes a Sotos syndrome-like phenotype and alters gene expression. Clin Genet 2010; 81: 56–63.
Tatton-Brown K, Cole TRP, Rahman N : Sotos syndrome; in Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K (eds): GeneReviews [Internet]. Seattle, WA: University of Washington 1993–2014 (17 December 2004 (updated 8 March 2012)).
Tatton-Brown K, Rahman N : Clinical features of NSD1-positive Sotos syndrome. Clin Dysmorphol 2004; 13: 199–204.
Baujat G, Cormier-Daire V : Sotos syndrome. Orphanet J Rare Dis 2007; 2: 36.
Bonaglia MC, Marelli S, Novara F et al: Genotype-phenotype relationship in three cases with overlapping 19p13.12 microdeletions. Eur J Hum Genet 2010; 18: 1302–1309.
Driller K, Pagenstecher A, Uhl M et al: Nuclear factor I X deficiency causes brain malformation and severe skeletal defects. Mol Cell Biol 2007; 27: 3855–3867.
Pietrobon D : Insights into migraine mechanisms and CaV2.1 calcium channel function from mouse models of familial hemiplegic migraine. J Physiol 2010; 588: 1871–1878.
Di Cristofori A, Fusi L, Gomitoni A, Grampa G, Bersano A : R583Q CACNA1A variant in SHM1 and ataxia: case report and literature update. J Headache Pain 2012; 13: 419–423.
Labrum RW, Rajakulendran S, Graves TD et al: Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing. J Med Genet 2009; 46: 786–791.
Spacey S : Episodic Ataxia Type 2; In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K (eds): GeneReviews [Internet]. Seattle (WA): University of Washington 1993–2014. (24 February 2003 (updated 8 December 2011)).
Riant F, Lescoat C, Vahedi K et al: Identification of CACNA1A large deletions in four patients with episodic ataxia. Neurogenetics 2009; 11: 101–106.
Wan J, Mamsa H, Johnston JL et al: Large genomic deletions in CACNA1A cause episodic ataxia type 2. Front Neurol 2011; 2: 51.
Cordaux R, Hedges DJ, Batzer MA : Retrotransposition of Alu elements: how many sources? Trends Genet 2004; 20: 464–467.
Acknowledgements
We thank the patients and their parents. M.K. is supported by a grant from the academic fund (‘Academisch Fonds’) of the Maastricht University Medical Centre. The DDD study presents independent research commissioned by the Health Innovation Challenge Fund [grant number HICF-1009-003], a parallel funding partnership between the Wellcome Trust and the Department of Health, and the Wellcome Trust Sanger Institute (grant number WT098051). The views expressed in this publication are those of the author(s) and not necessarily those of the Wellcome Trust or the Department of Health. The study has UK Research Ethics Committee approval (10/H0305/83, granted by the Cambridge South REC, and GEN/284/12 granted by the Republic of Ireland REC). The research team acknowledges the support of the National Institute for Health Research, through the Comprehensive Clinical Research Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Klaassens, M., Morrogh, D., Rosser, E. et al. Malan syndrome: Sotos-like overgrowth with de novo NFIX sequence variants and deletions in six new patients and a review of the literature. Eur J Hum Genet 23, 610–615 (2015). https://doi.org/10.1038/ejhg.2014.162
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2014.162
This article is cited by
-
Erythroid lineage chromatin accessibility maps facilitate identification and validation of NFIX as a fetal hemoglobin repressor
Communications Biology (2023)
-
A deep phenotyping experience: up to date in management and diagnosis of Malan syndrome in a single center surveillance report
Orphanet Journal of Rare Diseases (2022)
-
Benefits of clinical criteria and high-throughput sequencing for diagnosing children with syndromic craniosynostosis
European Journal of Human Genetics (2021)
-
Granule neuron precursor cell proliferation is regulated by NFIX and intersectin 1 during postnatal cerebellar development
Brain Structure and Function (2019)
-
19p13 microduplications encompassing NFIX are responsible for intellectual disability, short stature and small head circumference
European Journal of Human Genetics (2018)