Abstract
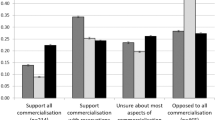
Although rarely acknowledged, a successful biobank is highly dependent on the support of the health professionals who assist the biobank in all aspects of its activities. In many cases, the lack of health professional support can be a limiting factor in the biobanking process of collecting and processing high-quality biospecimens. The aim of this study was to determine the attitudes of health professionals towards cancer biobanking. Using a 5-point Likert scale questionnaire, important aspects of biobanking, including accrual, quality, knowledge, responsiveness, impact, access, trust, governance and accreditation, were investigated. In total, 95 of 124 health and medical practitioners who were approached participated in this study (77% response rate). Health professionals in general supported the aims of biobanking with 56% of participants showing willingness to create a biobank and recruit donors (accrual), 85% understanding the importance in the storage and distribution of biospecimens (quality), 88% having an appreciation for the role of a biobank in furthering cancer research (knowledge), 70% showing awareness of the use of biospecimens in future research initiatives (responsiveness) and 73% demonstrating support for a biobank with proper control, authority and credibility measures in place (governance and accreditation). Overall, provided that proper information about the activities of the biobank and researcher access was transparent, health professionals were very willing to support cancer biobanking. These findings may assist in developing strategies for the establishment and maintenance of biobanks and aid the implementation of more effective policies and procedures to embed biobanking into routine hospital practices.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barnes RO, Parisien M, Murphy LC, Watson PH : Influence of evolution in tumor biobanking on the interpretation of translational research. Cancer Epidemiol Biomarkers Prev 2008; 17: 3344–3350.
Otlowski MFA, Nicol D, Stranger MJA : Biobanks information paper 2010. J Law Info Sci 2010; 20: 97–227.
Riegman PH, Morente MM, Betsou F, de Blasio P, Geary P, Marble Arch International Working Group on Biobanking for Biomedical Research: Biobanking for better healthcare. Mol Oncol 2008; 2: 213–222.
Wyld L, Smith S, Hawkins NJ, Long J, Ward RL : Introducing research initiatives into healthcare: what do doctors think? Biopreserv Biobank 2014; 12: 91–98.
Nicol D, Critchley C : Benefit sharing and biobanking in Australia. Public Underst Sci 2012; 21: 534–555.
O'Doherty KC, Hawkins A : Structuring public engagement for effective input in policy development on human tissue biobanking. Public Health Genomics 2010; 13: 197–206.
Whitley EA, Kanellopoulou N, Kaye J : Consent and research governance in biobanks: evidence from focus groups with medical researchers. Public Health Genomics 2012; 15: 232–242.
Garcia DL, Bracci PM, Guevarra DM, Sieffert N : International Society for Biological and Environmental Repositories (ISBER) tools for the biobanking community. Biopreserv Biobank 2014; 12: 435–436.
Vaught J, Campbell LD, Betsou F et al: The ISBER best practices: insight from the editors of the third edition. Biopreserv Biobank 2012; 10: 76–78.
Ford E, Jenkins V, Fallowfield L, Stuart N, Farewell D, Farewell V : Clinicians' attitudes towards clinical trials of cancer therapy. Br J Cancer 2011; 104: 1535–1543.
Caldwell PH, Craig JC, Butow PN : Barriers to Australian physicians' and paediatricians' involvement in randomised controlled trials. Med J Australia 2005; 182: 59–65.
Fallowfield L, Ratcliffe D, Souhami R : Clinicians' attitudes to clinical trials of cancer therapy. Eur J Cancer 1997; 33: 2221–2229.
Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F : Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer 2002; 95: 1577–1583.
Ulrich CM, James JL, Walker EM et al: RTOG physician and research associate attitudes, beliefs and practices regarding clinical trials: implications for improving patient recruitment. Contemp Clin Trials 2010; 31: 221–228.
Leiman DA, Lorenzi NM, Wyatt JC, Doney AS, Rosenbloom ST : US and Scottish health professionals' attitudes toward DNA biobanking. J Am Med Inform Assoc 2008; 15: 357–362.
Meulenkamp TM, Gevers SJ, Bovenberg JA, Smets EM : Researchers' opinions towards the communication of results of biobank research: a survey study. Eur J Hum Genet 2012; 20: 258–262.
Johnsson L, Helgesson G, Rafnar T et al: Hypothetical and factual willingness to participate in biobank research. Eur J Hum Genet 2010; 18: 1261–1264.
Likert R : A technique for the measurement of attitudes. Arch Psychol 1932; 22: 55.
Harris P, Barton M : Research Strategy for South Western Sydney Local Health District 2012-2021. South Western Sydney Local Health District 2012, Available from http://www.swslhd.nsw.gov.au/pdfs/SWSLHD_Research_Strategy_2012.pdf.
Jenkins V, Farewell D, Batt L et al: The attitudes of 1066 patients with cancer towards participation in randomised clinical trials. Br J Cancer 2010; 103: 1801–1807.
Luque JS, Quinn GP, Montel-Ishino FA et al: Formative research on perceptions of biobanking: what community members think. J Cancer Educ 2012; 27: 91–99.
Beskow LM, Smolek SJ : Prospective biorepository participants' perspectives on access to research results. J Empir Res Hum Res Ethics 2009; 4: 99–111.
Allen NL, Karlson EW, Malspeis S, Lu B, Seidman CE, Lehmann LS : Biobank participants' preferences for disclosure of genetic research results: perspectives from the OurGenes, OurHealth, OurCommunity project. Mayo Clin Proc 2014; 89: 738–746.
Cassa CA, Savage SK, Taylor PL, Green RC, McGuire AL, Mandl KD : Disclosing pathogenic genetic variants to research participants: quantifying an emerging ethical responsibility. Genome Res 2012; 22: 421–428.
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R : Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999; 52: 1143–1156.
Ajzen I : The theory of planned behaviour: reactions and reflections. Psychol Health 2011; 26: 1113–1127.
Allen P, Bennett K : PASW Statistics by SPSS: A Practical Guide Vol. 18. South Melbourne: Cengage Learning, 2010.
Acknowledgements
This work was supported by the University of Western Sydney and the University of New South Wales. Funding sources include the Cancer Institute New South Wales for CONCERT. We thank Norbert Kienzle and Jennifer O’Shea for their thoughtful contributions to the study and Björn Espedido and Kieran Scott for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Caixeiro, N., Byun, H., Descallar, J. et al. Health professionals’ opinions on supporting a cancer biobank: identification of barriers to combat biobanking pitfalls. Eur J Hum Genet 24, 626–632 (2016). https://doi.org/10.1038/ejhg.2015.191
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.191