Abstract
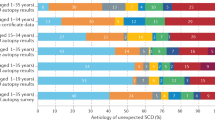
Sudden infant death syndrome (SIDS) is the most frequent manner of post-perinatal death among infants. One of the suggested causes of the syndrome is inherited cardiac diseases, mainly channelopathies, that can trigger arrhythmias and sudden death. The purpose of this study was to investigate cases of sudden unexpected death in infancy (SUDI) for potential causative variants in 100 cardiac-associated genes. We investigated 47 SUDI cases of which 38 had previously been screened for variants in RYR2, KCNQ1, KCNH2 and SCN5A. Using the Haloplex Target Enrichment System (Agilent) and next-generation sequencing (NGS), the coding regions of 100 genes associated with inherited channelopathies and cardiomyopathies were captured and sequenced on the Illumina MiSeq platform. Sixteen (34%) of the SUDI cases had variants with likely functional effects, based on conservation, computational prediction and allele frequency, in one or more of the genes screened. The possible effects of the variants were not verified with family or functional studies. Eight (17%) of the SUDI cases had variants in genes affecting ion channel functions. The remaining eight cases had variants in genes associated with cardiomyopathies. In total, one third of the SUDI victims in a forensic setting had variants with likely functional effect that presumably contributed to the cause of death. The results support the assumption that channelopathies are important causes of SUDI. Thus, analysis of genes associated with cardiac diseases in SUDI victims is important in the forensic setting and a valuable supplement to the clinical investigation in all cases of sudden death.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Krous HF, Beckwith JB, Byard RW et al: Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 2004; 114: 234–238.
Changing concepts of sudden infant death syndrome: implications for infant sleeping environment and sleep position. American Academy of Pediatrics. Task Force on Infant Sleep Position and Sudden Infant Death Syndrome. Pediatrics 2000; 105 (3 Pt 1): 650–656.
Moon RY, Horne RS, Hauck FR : Sudden infant death syndrome. Lancet 2007; 370: 1578–1587.
Winkel BG, Holst AG, Theilade J et al: Sudden unexpected death in infancy in Denmark. Scand Cardiovasc J 2011; 45: 14–20.
Millat G, Kugener B, Chevalier P et al: Contribution of long-QT syndrome genetic variants in sudden infant death syndrome. Pediatr Cardiol 2009; 30: 502–509.
Arnestad M, Crotti L, Rognum TO et al: Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation 2007; 115: 361–367.
Larsen MK, Berge KE, Leren TP et al: Postmortem genetic testing of the ryanodine receptor 2 (RYR2) gene in a cohort of sudden unexplained death cases. Int J Legal Med 2012; 127: 139–144.
Schwartz PJ, Crotti L, Insolia R : Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol 2012; 5: 868–877.
Crotti L, Johnson CN, Graf E et al: Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 2013; 127: 1009–1017.
Brion M, Allegue C, Santori M et al: Sarcomeric gene mutations in sudden infant death syndrome (SIDS). Forensic Sci Int 2012; 219: 278–281.
Larsen MK. : Sudden unexpected death and genetic heart disease: a molecular autopsy study. PhD dissertation, Aarhus University: Denmark, 2012.
Vege Å, Rognum T, Løberg E et alDiagnosis of sudden infant death in the Nordic countries since 1970, revised (1995); in: Rognum TO (ed): Sudden Infant Death Syndrome: New Trends in the Nineties. Scandinavian University Press, 1995, pp 67–69.
OMIM Online Mendelian Inheritance in Man. Baltimore, MD: McKusick-Nathans Institute of Genetic Medicine, John Hopkins University.
Kent W, Sugnet C, Furey T et al: The human genome browser at USCS. Genome Res. 2002; 12: 996–1006.
Li H, Durbin R : Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009; 25: 1754–1760.
Li H, Handsaker B, Wysoker A et al1000 Genome Project Data Processing Subgroup: The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009; 25: 2078–2079.
ESP. Exome Variant Server [updated 2014/01/02/10:04:37]. Available from http://evs.gs.washington.edu/EVS/.
NCBI. National Center for Biotechnology Information [updated 2014/01/02/10:23:41]. Available from http://www.ncbi.nlm.nih.gov/.
Stenson P, Ball E, Mort M et al: The Human Gene Mutation Database (HGMD). Hum Mutat 2003; 21: 577–581.
Hertz CL, Christiansen SL, Ferrero-Miliani L et al: Next-generation sequencing of 34 genes in sudden unexplained death victims in forensics and in patients with channelopathic cardiac diseases. Int J Legal Med 2014; 129: 793–800.
Richards S, Aziz N, Bale S et al: Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–423.
Ng D, Johnston JJ, Teer JK et alNIH Intramural Sequencing Center (NISC) Comparative Sequencing Program: Interpreting secondary cardiac disease variants in an exome cohort. Circ Cardiovasc Genet 2013; 6: 337–346.
Dorschner MO, Amendola LM, Turner EH et alNational Heart, Lung, and Blood Institute Grand Opportunity Exome Sequencing Project: Actionable, pathogenic incidental findings in 1,000 participants' exomes. Am J Hum Genet 2013; 93: 631–640.
Mathieson I, McVean G : Differential confounding of rare and common variants in spatially structured populations. Nat Genet. 2012; 44: 243–246.
Lohmueller KE, Sparso T, Li Q et al: Whole-exome sequencing of 2,000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am J Hum Genet 2013; 93: 1072–1086.
R Core Team. R: A Language and Environment for Statistical Computing 2014, Available from http://www.R-project.org.
Huber W, Carey VJ, Gentleman R et al: Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 2015; 12: 115–121.
Gentleman RC, Carey VJ, Bates DM et al: Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP : TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002; 109: 397–407.
Nilius B, Prenen J, Tang J et al: Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 2005; 280: 6423–6433.
Le Scouarnec S, Karakachoff M, Gourraud JB et al: Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Hum Mol Genet 2015; 24: 2757–2763.
Arndt AK, Schafer S, Drenckhahn JD et al: Fine mapping of the 1p36 deletion syndrome identifies mutation of PRDM16 as a cause of cardiomyopathy. Am J Hum Genet 2013; 93: 67–77.
Xing Y, Ichida F, Matsuoka T et al: Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab 2006; 88: 71–77.
Campuzano O, Allegue C, Sarquella-Brugada G et al: The role of clinical, genetic and segregation evaluation in sudden infant death. Forensic Sci Int 2014; 242: 9–15.
Cerrone M, Noorman M, Lin X et al: Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 2012; 95: 460–468.
Rizzo S, Lodder EM, Verkerk AO et al: Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012; 95: 409–418.
Zhang Q, Deng C, Rao F et al: Silencing of desmoplakin decreases connexin43/Nav1.5 expression and sodium current in HL1 cardiomyocytes. Mole Med Rep 2013; 8: 780–786.
Cerrone M, Lin X, Zhang M et al: Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014; 129: 1092–1103.
Fleming PJ, Blair PS, Pease A : Sudden unexpected death in infancy: aetiology, pathophysiology, epidemiology and prevention in 2015. Arch Dis Childh 2015, e-pub ahead of print 19 February 2015 doi:10.1136/archdischild-2014-306424.
Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ : Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet 2006; 367: 314–319.
Blair PS, Sidebotham P, Evason-Coombe C, Edmonds M, Heckstall-Smith EM, Fleming P : Hazardous cosleeping environments and risk factors amenable to change: case-control study of SIDS in south west England. BMJ 2009; 339: b3666.
Mollborg P, Wennergren G, Almqvist P, Alm B : Bed sharing is more common in sudden infant death syndrome than in explained sudden unexpected deaths in infancy. Acta Paediatr 2015; 104: 777–783.
Mohler PJ, Schott J-J, Gramolini AO et al: Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003; 421: 634–639.
Roux-Buisson N, Gandjbakhch E, Donal E et al: Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: results of a systematic screening. Heart Rhythm 2014; 11: 1999–2009.
Granados-Riveron JT, Ghosh TK, Pope M et al: Alpha-cardiac myosin heavy chain (MYH6) mutations affecting myofibril formation are associated with congenital heart defects. Hum Mol Genet 2010; 19: 4007–4016.
Li FY, El-Hattab AW, Bawle EV et al: Molecular spectrum of SLC22A5 (OCTN2) gene mutations detected in 143 subjects evaluated for systemic carnitine deficiency. Hum Mutat 2010; 31: E1632–E1651.
Liu H, El Zein L, Kruse M et al: Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 2010; 3: 374–385.
Stallmeyer B, Zumhagen S, Denjoy I et al: Mutational spectrum in the Ca(2+)—activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 2012; 33: 109–117.
Acknowledgements
We thank Francisc-Raul Kantor for bioinformatics support. For the screening of variants among Danish controls, we thank LuCamp, The Lundbeck Foundation Centre for Applied Medical Genomics in Personalized Disease Prediction, Prevention, and Care (www.lucamp.org) and the Novo Nordisk Foundation Center for Basic Metabolic Research, an independent Research Centre at the University of Copenhagen partially supported by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk). This work was supported by Ellen and Aage Andersen’s Foundation and Arvid Nilssons’s Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Hertz, C., Christiansen, S., Larsen, M. et al. Genetic investigations of sudden unexpected deaths in infancy using next-generation sequencing of 100 genes associated with cardiac diseases. Eur J Hum Genet 24, 817–822 (2016). https://doi.org/10.1038/ejhg.2015.198
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.198
This article is cited by
-
Investigating cardiac genetic background in sudden infant death syndrome (SIDS)
International Journal of Legal Medicine (2024)
-
Value of next-generation sequencing in inherited arrhythmia syndromes
International Journal of Arrhythmia (2023)
-
HPO-driven virtual gene panel: a new efficient approach in molecular autopsy of sudden unexplained death
BMC Medical Genomics (2021)
-
Postmortale molekulargenetische Untersuchungen (molekulare Autopsie) bei kardiovaskulären und bei ungeklärten Todesfällen
Der Kardiologe (2021)
-
Genetic investigations of 100 inherited cardiac disease-related genes in deceased individuals with schizophrenia
International Journal of Legal Medicine (2021)