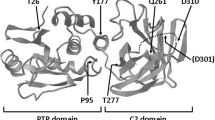
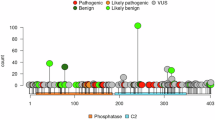
Abstract
PTEN hamartoma tumour syndrome (PHTS) is caused by heterozygous variants in PTEN and is characterised by tumour predisposition, macrocephaly, and cognition impairment. Bi-allelic loss of PTEN activity has not been reported so far and animal models suggest that bi-allelic loss of PTEN activity is embryonically lethal. Here, we report the identification of a novel homozygous variant in PTEN, NM_000314.4; c.545T>C; p.Leu182Ser, in two adolescent siblings with severe macrocephaly and mild intellectual disability. The variant is predicted to be damaging and is associated with significantly increased phospho-S6 downstream of PTEN. The absence of tumours in the two homozygous siblings as well as lack of symptoms of PHTS in the heterozygous carriers of the family suggest that this particular variant is functionally hypomorphic rather than deleterious.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Teresi RE, Zbuk KM, Pezzolesi MG, Waite KA, Eng C : Cowden syndrome-affected patients with PTEN promoter mutations demonstrate abnormal protein translation. Am J Hum Genet 2007; 81: 756–767.
Tan MH, Mester J, Peterson C et al: A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet 2011; 88: 42–56.
Mester J, Eng C : When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet 2013; 163C: 114–121.
Hanssen AM, Fryns JP : Cowden syndrome. J Med Genet 1995; 32: 117–119.
Parisi MA, Dinulos MB, Leppig KA, Sybert VP, Eng C, Hudgins L : The spectrum and evolution of phenotypic findings in PTEN mutation positive cases of Bannayan-Riley-Ruvalcaba syndrome. J Med Genet 2001; 38: 52–58.
Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E : Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 2013; 105: 1607–1616.
Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW : PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci 2010; 30: 9306–9315.
Maehama T, Dixon JE : The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–13378.
Myers MP, Stolarov JP, Eng C et al: P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 1997; 94: 9052–9057.
Luo X, Park KK : Neuron-intrinsic inhibitors of axon regeneration: PTEN and SOCS3. Int Rev Neurobiol 2012; 105: 141–173.
Kim JY, Duan X, Liu CY et al: DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 2009; 63: 761–773.
Stiles B, Groszer M, Wang S, Jiao J, Wu H : PTENless means more. Dev Biol 2004; 273: 175–184.
Kwon CH, Luikart BW, Powell CM et al: Pten regulates neuronal arborization and social interaction in mice. Neuron 2006; 50: 377–388.
Abou Jamra R, Wohlfart S, Zweier M et al: Homozygosity mapping in 64 Syrian consanguineous families with non-specific intellectual disability reveals 11 novel loci and high heterogeneity. Eur J Hum Genet 2011; 19: 1161–1166.
Murakami Y, Tawamie H, Maeda Y et al: Null mutation in PGAP1 impairing Gpi-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genet 2014; 10: e1004320.
Hartel C, Bachmann S, Bonnemann C, Meinecke P, Sperner J : Familial megalencephaly with dilated Virchow-Robin spaces in magnetic resonance imaging: an autosomal recessive trait? Clin Dysmorphol 2005; 14: 31–34.
Rosivatz E, Matthews JG, McDonald NQ et al: A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN). ACS Chem Biol 2006; 1: 780–790.
Li Y, Prasad A, Jia Y et al: Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood 2011; 117: 6702–6713.
Spinelli L, Lindsay YE, Leslie NR : PTEN inhibitors: an evaluation of current compounds. Adv Biol Regul 2015; 57: 102–111.
Heindl M, Händel N, Ngeow J et al: Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterology 2012; 142: 1093–1096.e1096.
Zhang XC, Piccini A, Myers MP, Van Aelst L, Tonks NK : Functional analysis of the protein phosphatase activity of PTEN. Biochem J 2012; 444: 457–464.
Kreis P, Leondaritis G, Lieberam I, Eickholt BJ : Subcellular targeting and dynamic regulation of PTEN: implications for neuronal cells and neurological disorders. Front Mol Neurosci 2014; 7: 23.
Busa T, Chabrol B, Perret O, Longy M, Philip N : Novel PTEN germline mutation in a family with mild phenotype: difficulties in genetic counseling. Gene 2013; 512: 194–197.
Liang H, He S, Yang J et al: PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab 2014; 19: 836–848.
Li Y, He L, Zeng N et al: Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling regulates mitochondrial biogenesis and respiration via estrogen-related receptor alpha (ERRalpha). J Biol Chem 2013; 288: 25007–25024.
Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H : Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 2015; 16: 178–187.
Lee JO, Yang H, Georgescu MM et al: Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 1999; 99: 323–334.
Acknowledgements
We thank the patients and their parents for participating in this study. We are grateful to Arif Ekici, Steffen Uebe, and Mandy Krumbiegel for support with the SNP arrays and whole exome sequencing, Farah Radwan, Angelika Diem, and Petra Rothe for excellent technical assistance, as well as Oliver Rompel for examining the MRI images. We thank Charis Eng and Stefan Aretz for helpful discussions and sharing unpublished results. This study was supported by grants from the Deutsche Forschungs-gemeinschaft to RAJ (AB393/2-2) and by Cancer Research UK grant number C38302/A12278, through the Oxford Cancer Research Centre Development Fund (OCRC0612-HU) to HHU. HHU and HC declare industry collaboration via the Oxford UCB target program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Schwerd, T., Khaled, A., Schürmann, M. et al. A recessive form of extreme macrocephaly and mild intellectual disability complements the spectrum of PTEN hamartoma tumour syndrome. Eur J Hum Genet 24, 889–894 (2016). https://doi.org/10.1038/ejhg.2015.209
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.209
This article is cited by
-
Differential cell cycle checkpoint evasion by PTEN germline mutations associated with dichotomous phenotypes of cancer versus autism spectrum disorder
Oncogene (2023)
-
Identification of differential gene expression profile from peripheral blood cells of military pilots with hypertension by RNA sequencing analysis
BMC Medical Genomics (2018)
-
Identification of mutations in the PI3K-AKT-mTOR signalling pathway in patients with macrocephaly and developmental delay and/or autism
Molecular Autism (2017)