Abstract
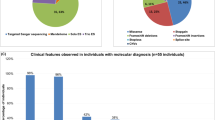
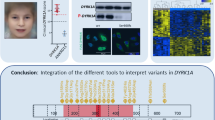
Dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1 A (DYRK1A ) is a highly conserved gene located in the Down syndrome critical region. It has an important role in early development and regulation of neuronal proliferation. Microdeletions of chromosome 21q22.12q22.3 that include DYRK1A (21q22.13) are rare and only a few pathogenic single-nucleotide variants (SNVs) in the DYRK1A gene have been described, so as of yet, the landscape of DYRK1A disruptions and their associated phenotype has not been fully explored. We have identified 14 individuals with de novo heterozygous variants of DYRK1A; five with microdeletions, three with small insertions or deletions (INDELs) and six with deleterious SNVs. The analysis of our cohort and comparison with published cases reveals that phenotypes are consistent among individuals with the 21q22.12q22.3 microdeletion and those with translocation, SNVs, or INDELs within DYRK1A. All individuals shared congenital microcephaly at birth, intellectual disability, developmental delay, severe speech impairment, short stature, and distinct facial features. The severity of the microcephaly varied from −2 SD to −5 SD. Seizures, structural brain abnormalities, eye defects, ataxia/broad-based gait, intrauterine growth restriction, minor skeletal abnormalities, and feeding difficulties were present in two-thirds of all affected individuals. Our study demonstrates that haploinsufficiency of DYRK1A results in a new recognizable syndrome, which should be considered in individuals with Angelman syndrome-like features and distinct facial features. Our report represents the largest cohort of individuals with DYRK1A disruptions to date, and is the first attempt to define consistent genotype–phenotype correlations among subjects with 21q22.13 microdeletions and DYRK1A SNVs or small INDELs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aranda S, Laguna A, de la Luna S : DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J 2011; 25: 449–462.
Becker W, Weber Y, Wetzel K, Eirmbter K, Tejedor FJ, Joost HG : Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J Biol Chem 1998; 273: 25893–25902.
Song WJ, Sternberg LR, Kasten-Sportes C et al: Isolation of human and murine homologues of the Drosophila minibrain gene: human homologue maps to 21q22.2 in the Down syndrome ‘critical region’. Genomics 1996; 38: 331–339.
Shapiro BL : The Down syndrome critical region. J Neural Transm Suppl 1999; 57: 41–60.
Tejedor FJ, Hammerle B : MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J 2011; 278: 223–235.
Becker W, Sippl W : Activation, regulation, and inhibition of DYRK1A. FEBS J 2011; 278: 246–256.
Fotaki V, Dierssen M, Alcantara S et al: Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol Cell Biol 2002; 22: 6636–6647.
Dierssen M, de Lagran MM : DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis. ScientificWorldJournal 2006; 6: 1911–1922.
Benavides-Piccione R, Dierssen M, Ballesteros-Yanez I et al: Alterations in the phenotype of neocortical pyramidal cells in the Dyrk1A+/− mouse. Neurobiol Dis 2005; 20: 115–122.
Moller RS, Kubart S, Hoeltzenbein M et al: Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am J Hum Genet 2008; 82: 1165–1170.
Courcet JB, Faivre L, Malzac P et al: The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J Med Genet 2012; 49: 731–736.
O'Roak BJ, Vives L, Girirajan S et al: Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485: 246–250.
van Bon BW, Hoischen A, Hehir-Kwa J et al: Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin Genet 2011; 79: 296–299.
Yamamoto T, Shimojima K, Nishizawa T, Matsuo M, Ito M, Imai K : Clinical manifestations of the deletion of Down syndrome critical region including DYRK1A and KCNJ6. Am J Med Genet A 2011; 155A: 113–119.
Fujita H, Torii C, Kosaki R et al: Microdeletion of the Down syndrome critical region at 21q22. Am J Med Genet A 2010; 152A: 950–953.
Oegema R, de Klein A, Verkerk AJ et al: Distinctive phenotypic abnormalities associated with submicroscopic 21q22 deletion including DYRK1A. Mol Syndromol 2010; 1: 113–120.
Valetto A, Orsini A, Bertini V et al: Molecular cytogenetic characterization of an interstitial deletion of chromosome 21 (21q22.13q22.3) in a patient with dysmorphic features, intellectual disability and severe generalized epilepsy. Eur J Med Genet 2012; 55: 362–366.
Strom SP, Gorin MB : Evaluation of autosomal dominant retinal dystrophy genes in an unaffected cohort suggests rare or private missense variants may often be benign. Molecular vision 2013; 19: 980–985.
Emsley P, Lohkamp B, Scott WG, Cowtan K : Features and development of Coot. Acta crystallogr Sect D Biol Crystallogr 2010; 66: 486–501.
Chen VB, Arendall WB 3rd, Headd JJ et al: MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr Sect D Biol Crystallogr 2010; 66: 12–21.
Endicott JA, Noble ME, Johnson LN : The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem 2012; 81: 587–613.
Kentrup H, Becker W, Heukelbach J et al: Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J Biol Chem 1996; 271: 3488–3495.
Wiechmann S, Czajkowska H, de Graaf K, Grotzinger J, Joost HG, Becker W : Unusual function of the activation loop in the protein kinase DYRK1A. Biochem Biophys Res Commun 2003; 302: 403–408.
Berto GE, Iobbi C, Camera P et al: The DCR protein TTC3 affects differentiation and Golgi compactness in neurons through specific actin-regulating pathways. PloS One 2014; 9: e93721.
Lochhead PA, Sibbet G, Morrice N, Cleghon V : Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell 2005; 121: 925–936.
Lepagnol-Bestel AM, Zvara A, Maussion G et al: DYRK1A interacts with the REST/NRSF-SWI/SNF chromatin remodelling complex to deregulate gene clusters involved in the neuronal phenotypic traits of Down syndrome. Hum mol Genet 2009; 18: 1405–1414.
Abekhoukh S, Planque C, Ripoll C et al: Dyrk1A, a serine/threonine kinase, is involved in ERK and Akt activation in the brain of hyperhomocysteinemic mice. Mol Neurobiol 2013; 47: 105–116.
Boku S, Nakagawa S, Takamura N et al: GDNF facilitates differentiation of the adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3. Biochem Biophys Res Commun 2013; 434: 779–784.
McMillan EL, Kamps AL, Lake SS, Svendsen CN, Bhattacharyya A : Gene expression changes in the MAPK pathway in both Fragile X and Down syndrome human neural progenitor cells. Am J Stem Cells 2012; 1: 154–162.
Kelly PA, Rahmani Z : DYRK1A enhances the mitogen-activated protein kinase cascade in PC12 cells by forming a complex with Ras, B-Raf, and MEK1. Mol Biol Cell 2005; 16: 3562–3573.
Lee Y, Ha J, Kim HJ et al: Negative feedback Inhibition of NFATc1 by DYRK1A regulates bone homeostasis. J Biol Chem 2009; 284: 33343–33351.
Walte A, Ruben K, Birner-Gruenberger R et al: Mechanism of dual specificity kinase activity of DYRK1A. FEBS J 2013; 280: 4495–4511.
Lund IV, Hu Y, Raol YH et al: BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal 2008; 1: ra9.
Chen CK, Bregere C, Paluch J, Lu JF, Dickman DK, Chang KT : Activity-dependent facilitation of Synaptojanin and synaptic vesicle recycling by the Minibrain kinase. Nat Commun 2014; 5: 4246.
Tejedor F, Zhu XR, Kaltenbach E et al: minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron 1995; 14: 287–301.
Laguna A, Barallobre MJ, Marchena MA et al: Triplication of DYRK1A causes retinal structural and functional alterations in Down syndrome. Hum Mol Genet 2013; 22: 2775–2784.
Rachdi L, Kariyawasam D, Guez F et al: Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass. Diabetologia 2014; 57: 960–969.
Hong SH, Lee KS, Kwak SJ et al: Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in Drosophila and mammals. PLoS Genet 2012; 8: e1002857.
Thevenon J, Callier P, Thauvin-Robinet C et al: De Novo 21q22.1q22.2 deletion including RUNX1 mimicking a congenital infection. Am J Med Genet A 2011; 155A: 126–129.
Izumi K, Brooks SS, Feret HA, Zackai EH : 1.9 Mb microdeletion of 21q22.11 within Braddock-Carey contiguous gene deletion syndrome region: dissecting the phenotype. Am J Med Genet A 2012; 158A: 1535–1541.
Lee H, Deignan JL, Dorrani N et al: Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 2014; 312: 1880–1887.
Wapner RJ, Martin CL, Levy B et al: Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med 2012; 367: 2175–2184.
Souchet B, Guedj F, Sahun I et al: Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis 2014; 69: 65–75.
De la Torre R, De Sola S, Pons M et al: Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res 2014; 58: 278–288.
Guedj F, Bianchi DW, Delabar JM : Prenatal treatment of Down syndrome: a reality? Curr Opin Obstet Gynecol 2014; 26: 92–103.
Acknowledgements
We would like to thank the families, the patients, and clinical staff at all locations for participation in this study. We are very grateful to Dr Xinmin Li, Jonathan David, Vanina Tomasina, Traci Toy, and Lynn Yang for their technical assistance on CMA, CES, and FISH analysis, and the UCLA-CGC Genomic Data Board members for their contribution to exome data interpretation. We thank Drs Aviv Paz and Thorsten Althoff for their assistance in structure analysis and figure generation, Dr Sulagna Saitta for useful discussion, and Dr Kevin M Squire for his critical reading of this manuscript. This work was partially presented at the 2014 American College of Medical Genetics and Genomics annual meeting, 26–29 March, Nashville, TN, USA, and the 35th David Smith Workshop on Morphogenesis and Dysmorphology at University of Madison, WI, 25–30 July, 2014. This study was supported by NIH grant GM078844 (JA), and the UCLA Department of Pathology translational research fund (FQ-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Ji, J., Lee, H., Argiropoulos, B. et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur J Hum Genet 23, 1473–1481 (2015). https://doi.org/10.1038/ejhg.2015.71
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.71
This article is cited by
-
Understanding the genetic mechanisms and cognitive impairments in Down syndrome: towards a holistic approach
Journal of Neurology (2024)
-
Regulation of fatty acid desaturase- and immunity gene-expression by mbk-1/DYRK1A in Caenorhabditis elegans
BMC Genomics (2022)
-
Social motivation a relative strength in DYRK1A syndrome on a background of significant speech and language impairments
European Journal of Human Genetics (2022)
-
The chromosome 21 kinase DYRK1A: emerging roles in cancer biology and potential as a therapeutic target
Oncogene (2022)
-
Impaired macroglial development and axonal conductivity contributes to the neuropathology of DYRK1A-related intellectual disability syndrome
Scientific Reports (2022)