Abstract
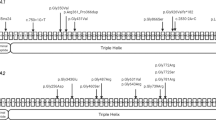
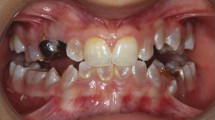
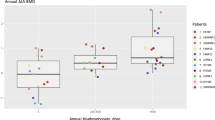
Osteogenesis imperfecta (OI) is a rare hereditary bone fragility disorder, caused by collagen I mutations in 90% of cases. There are no comprehensive genotype–phenotype studies on >100 families outside North America, and no population-based studies determining the genetic epidemiology of OI. Here, detailed clinical phenotypes were recorded, and the COL1A1 and COL1A2 genes were analyzed in 164 Swedish OI families (223 individuals). Averages for bone mineral density (BMD), height and yearly fracture rate were calculated and related to OI and mutation type. N-terminal helical mutations in both the α1- and α2-chains were associated with the absence of dentinogenesis imperfecta (P<0.0001 vs 0.0049), while only those in the α1-chain were associated with blue sclera (P=0.0110). Comparing glycine with serine substitutions, α1-alterations were associated with more severe phenotype (P=0.0031). Individuals with type I OI caused by qualitative vs quantitative mutations were shorter (P<0.0001), but did not differ considering fractures or BMD. The children in this cohort were estimated to represent >95% of the complete Swedish pediatric OI population. The prevalence of OI types I, III, and IV was 5.16, 0.89, and 1.35/100 000, respectively (7.40/100 000 overall), corresponding to what has been estimated but not unequivocally proven in any population. Collagen I mutation analysis was performed in the family of 97% of known cases, with causative mutations found in 87%. Qualitative mutations caused 32% of OI type I. The data reported here may be helpful to predict phenotype, and describes for the first time the genetic epidemiology in >95% of an entire OI population.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
16 July 2015
Supplementary Tables 1 and 2 have been replaced since online publication. A corrigendum appears in this issue
References
Barnes AM, Chang W, Morello R et al: Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 2006; 355: 2757–2764.
Baldridge D, Schwarze U, Morello R et al: CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat 2008; 29: 1435–1442.
van Dijk FS, Nesbitt IM, Zwikstra EH et al: PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet 2009; 85: 521–527.
Christiansen HE, Schwarze U, Pyott SM et al: Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet 2010; 86: 389–398.
Alanay Y, Avaygan H, Camacho N et al: Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2010; 86: 551–559.
Homan EP, Rauch F, Grafe I et al: Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res 2011; 26: 2798–2803.
Kelley BP, Malfait F, Bonafe L et al: Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res 2011; 26: 666–672.
Puig-Hervas MT, Temtamy S, Aglan M et al: Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome—osteogenesis imperfecta phenotypic spectrum. Hum Mutat 2012; 33: 1444–1449.
Semler O, Garbes L, Keupp K et al: A mutation in the 5'-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet 2012; 91: 349–357.
Shaheen R, Alazami AM, Alshammari MJ et al: Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J Med Genet 2012; 49: 630–635.
Laine CM, Joeng KS, Campeau PM et al: WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med 2013; 368: 1809–1816.
Symoens S, Malfait F, DH S et al: Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J Rare Dis 2013; 8: 154.
van Dijk FS, Zillikens MC, Micha D et al: PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med 2013; 369: 1529–1536.
Sillence DO, Senn A, Danks DM : Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 1979; 16: 101–116.
Marini JC, Forlino A, Cabral WA et al: Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 2007; 28: 209–221.
Rauch F, Lalic L, Roughley P, Glorieux FH : Genotype-phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet 2010; 18: 642–647.
Dalgleish R : The human type I collagen mutation database. Nucleic Acids Res 1997; 25: 181–187.
Dalgleish R : The Human Collagen Mutation Database 1998. Nucleic Acids Res 1998; 26: 253–255.
Dalgleish R . http://www.le.ac.uk/ge/collagen/).
Steiner RD, Adsit J, Basel D : COL1A1/2-related osteogenesis imperfecta; in: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K (eds): GeneReviews. Seattle, USA: University of Washington, 1993.
Astrom E, Soderhall S : Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatr 1998; 87: 64–68.
http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish, 2014.
Korkko J, Ala-Kokko L, De Paepe A, Nuytinck L, Earley J, Prockop DJ : Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet 1998; 62: 98–110.
Lindahl K, Barnes AM, Fratzl-Zelman N et al: COL1 C-propeptide cleavage site mutations cause high bone mass osteogenesis imperfecta. Hum Mutat 2011; 32: 598–609.
Exome Aggregation Consortium (ExAC): Cambridge, MA. Available at http://exac.broadinstitute.org (last accessed March 2015).
Adzhubei I, Jordan DM, Sunyaev SR : Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 2013; Chapter 7: Unit7 20.
Kumar P, Henikoff S, Ng PC : Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081.
Hartikka H, Kuurila K, Korkko J et al: Lack of correlation between the type of COL1A1 or COL1A2 mutation and hearing loss in osteogenesis imperfecta patients. Hum Mutat 2004; 24: 147–154.
Pollitt R, McMahon R, Nunn J et al: Mutation analysis of COL1A1 and COL1A2 in patients diagnosed with osteogenesis imperfecta type I-IV. Hum Mutat 2006; 27: 716.
Sillence DO, Rimoin DL : Classification of osteogenesis imperfect. Lancet 1978; 1: 1041–1042.
Orwoll ES, Shapiro J, Veith S et al: Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 2014; 124: 491–498.
Schorderet DF, Gartler SM : Analysis of CpG suppression in methylated and nonmethylated species. Proc Natl Acad Sci USA 1992; 89: 957–961.
Acknowledgements
We thank all of the individuals with OI who participated in this study. Furthermore, we thank Anna-Lena Johansson, Elin Carlsson, and Catharina Kumlien for skillful technical assistance. Funding was received from the Swedish research council, and from the Crown-princess Lovisa, Norrbacka Eugenia, Promobilia, RBU, and Sunnerdahls foundations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Lindahl, K., Åström, E., Rubin, CJ. et al. Genetic epidemiology, prevalence, and genotype–phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur J Hum Genet 23, 1042–1050 (2015). https://doi.org/10.1038/ejhg.2015.81
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2015.81
This article is cited by
-
Mesenchymal stem cells in the treatment of osteogenesis imperfecta
Cell Regeneration (2023)
-
The patient clinical journey and socioeconomic impact of osteogenesis imperfecta: a systematic scoping review
Orphanet Journal of Rare Diseases (2023)
-
Transition from Pediatric to Adult Health Care in Osteogenesis Imperfecta
Current Osteoporosis Reports (2023)
-
Genotype–phenotype relationship and comparison between eastern and western patients with osteogenesis imperfecta
Journal of Endocrinological Investigation (2023)
-
Patient-reported outcomes in a Chinese cohort of osteogenesis imperfecta unveil psycho-physical stratifications associated with clinical manifestations
Orphanet Journal of Rare Diseases (2022)