Abstract
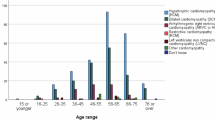
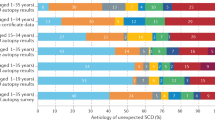
In forensic medicine, one-third of the sudden deaths remain unexplained after medico-legal autopsy. A major proportion of these sudden unexplained deaths (SUD) are considered to be caused by inherited cardiac diseases. Sudden cardiac death (SCD) may be the first manifestation of these diseases. The purpose of this study was to explore the yield of next-generation sequencing of genes associated with SCD in a cohort of SUD victims. We investigated 100 genes associated with cardiac diseases in 61 young (1–50 years) SUD cases. DNA was captured with the Haloplex target enrichment system and sequenced using an Illumina MiSeq. The identified genetic variants were evaluated and classified as likely, unknown or unlikely to have a functional effect. The criteria for this classification were based on the literature, databases, conservation and prediction of the effect of the variant. We found that 21 (34%) individuals carried variants with a likely functional effect. Ten (40%) of these variants were located in genes associated with cardiomyopathies and 15 (60%) of the variants in genes associated with cardiac channelopathies. Nineteen individuals carried variants with unknown functional effect. Our findings indicate that broad genetic investigation of SUD victims increases the diagnostic outcome, and the investigation should comprise genes involved in both cardiomyopathies and cardiac channelopathies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Winkel BG, Holst AG, Theilade J et al: Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J 2011; 32: 983–990.
Risgaard B, Winkel BG, Jabbari R et al: Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in Denmark. Circ Arrhythm Electrophysiol 2014; 7: 205–211.
Cerrone M, Lin X, Zhang M et al: Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014; 129: 1092–1103.
Zhang M, Tavora F, Oliveira JB et al: PKP2 mutations in sudden death from arrhythmogenic right ventricular cardiomyopathy (ARVC) and sudden unexpected death with negative autopsy (SUDNA). Circ J 2012; 76: 189–194.
Zhang Q, Deng C, Rao F et al: Silencing of desmoplakin decreases connexin43/Nav1.5 expression and sodium current in HL1 cardiomyocytes. Mol Med Rep 2013; 8: 780–786.
Rizzo S, Lodder EM, Verkerk AO et al: Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012; 95: 409–418.
Skinner JR, Crawford J, Smith W et al: Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm 2011; 8: 412–419.
Winkel BG, Larsen MK, Berge KE et al: The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol 2012; 23: 1092–1098.
Tester DJ, Medeiros-Domingo A, Will ML, Haglund CM, Ackerman MJ : Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 2012; 87: 524–539.
Kauferstein S, Kiehne N, Jenewein T et al: Genetic analysis of sudden unexplained death: a multidisciplinary approach. Forensic Sci Int 2013; 229: 122–127.
Hertz CL, Christiansen SL, Ferrero-Miliani L et al: Next-generation sequencing of 34 genes in sudden unexplained death victims in forensics and in patients with channelopathic cardiac diseases. Int J Legal Med 2015; 129: 793–800.
Narula N, Tester DJ, Paulmichl A, Maleszewski JJ, Ackerman MJ : Post-mortem Whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: a case series. Pediatr Cardiol 2015; 36: 768–778.
Bagnall RD, Das KJ, Duflou J, Semsarian C : Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm 2014; 11: 655–662.
Loporcaro CG, Tester DJ, Maleszewski JJ, Kruisselbrink T, Ackerman MJ : Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next-generation sequencing. Arch Pathol Lab Med 2014; 138: 1083–1089.
Hertz CL, Christiansen SL, Ferrero-Miliani L et al: Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int J Legal Med 2015; 130: 91–102.
Hertz CL, Christiansen SL, Larsen MK et al: Genetic investigations of sudden unexpected deaths in infancy using next-generation sequencing of 100 genes associated with cardiac diseases. Eur J Hum Genet 2015; 24: 817–822.
Stenson PD, Ball EV, Mort M et al: Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 2003; 21: 577–581.
Richards S, Aziz N, Bale S et al: Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–423.
Ng D, Johnston JJ, Teer JK et al: Interpreting secondary cardiac disease variants in an exome cohort. Circ Cardiovasc Genet 2013; 6: 337–346.
Dorschner MO, Amendola LM, Turner EH et al: Actionable, pathogenic incidental findings in 1000 participants’ exomes. Am J Hum Genet 2013; 93: 631–640.
Mathieson I, McVean G : Differential confounding of rare and common variants in spatially structured populations. Nat Genet 2012; 44: 243–246.
Lohmueller KE, Sparso T, Li Q et al: Whole-exome sequencing of 2000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am J Hum Genet 2013; 93: 1072–1086.
R Core Team. R: A language and environment for statistical computing, 2014.
Gerull B, Heuser A, Wichter T et al: Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 2004; 36: 1162–1164.
Christensen AH, Benn M, Tybjaerg-Hansen A, Haunso S, Svendsen JH : Missense variants in plakophilin-2 in arrhythmogenic right ventricular cardiomyopathy patients—disease-causing or innocent bystanders? Cardiology 2010; 115: 148–154.
Brion M, Allegue C, Santori M et al: Sarcomeric gene mutations in sudden infant death syndrome (SIDS). Forensic Sci Int 2012; 219: 278–281.
Zimmerman RS, Cox S, Lakdawala NK et al: A novel custom resequencing array for dilated cardiomyopathy. Genet Med 2010; 12: 268–278.
Morita H, Rehm HL, Menesses A et al: Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med 2008; 358: 1899–1908.
Andreasen C, Nielsen JB, Refsgaard L et al: New population-based exome data are questioning the pathogenicity of previously cardiomyopathy-associated genetic variants. Eur J Hum Genet 2013; 21: 918–928.
Roberts JD, Veinot JP, Rutberg J, Gollob MH : Inherited cardiomyopathies mimicking arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol 2010; 19: 316–320.
Laursen TM, Nordentoft M, Mortensen PB : Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014; 10: 425–448.
Sweeting J, Duflou J, Semsarian C : Postmortem analysis of cardiovascular deaths in schizophrenia: a 10-year review. Schizophr Res 2013; 150: 398–403.
Weeke P, Jensen A, Folke F et al: Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther 2012; 92: 72–79.
Fanoe S, Kristensen D, Fink-Jensen A et al: Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J 2014; 35: 1306–1315.
Straus SM, Bleumink GS, Dieleman JP et al: Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004; 164: 1293–1297.
Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT : Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry 2001; 58: 1161–1167.
Ray WA, Chung CP, Murray KT, Hall K, Stein CM : Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–235.
Nashef L : Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997; 38 (Suppl 11): S6–S8.
Holst AG, Winkel BG, Risgaard B et al: Epilepsy and risk of death and sudden unexpected death in the young: a nationwide study. Epilepsia 2013; 54: 1613–1620.
Partemi S, Vidal MC, Striano P et al: Genetic and forensic implications in epilepsy and cardiac arrhythmias: a case series. Int J Legal Med 2015; 129: 495–504.
Verma A, Kumar A : Sudden unexpected death in epilepsy: some approaches for its prevention and medico-legal consideration. Acta Neurol Belg 2015; 115: 207–212.
Bermeo-Ovalle AC, Kennedy JD, Schuele SU : Cardiac and Autonomic Mechanisms Contributing to SUDEP. J Clin Neurophysiol 2015; 32: 21–29.
Tu E, Bagnall RD, Duflou J, Semsarian C : Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol 2011; 21: 201–208.
Ponting CP : Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem Sci 2000; 25: 48–50.
Burashnikov E, Pfeiffer R, Barajas-Martinez H et al: Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 2010; 7: 1872–1882.
Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L : Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. J Am Coll Cardiol 1999; 34: 823–829.
Van Driest SL, Vasile VC, Ommen SR et al: Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44: 1903–1910.
Tester DJ, Will ML, Haglund CM, Ackerman MJ : Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005; 2: 507–517.
Zareba W, Moss AJ, Sheu G et al: Location of mutation in the KCNQ1 and phenotypic presentation of long QT syndrome. J Cardiovasc Electrophysiol 2003; 14: 1149–1153.
van Tintelen JP, Entius MM, Bhuiyan ZA et al: Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2006; 113: 1650–1658.
Shy D, Gillet L, Ogrodnik J et al: PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation 2014; 130: 147–160.
Kapplinger JD, Tester DJ, Salisbury BA et al: Spectrum and prevalence of mutations from the first 2500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 2009; 6: 1297–1303.
Acknowledgements
We thank Francisc-Raul Kantor and Carina Grøntved Jønck for bioinformatics support, and Eva Tonnesen for technical support in the laboratory. For the screening of variants among Danish controls, we thank LuCamp, The Lundbeck Foundation Centre for Applied Medical Genomics in Personalized Disease Prediction, Prevention and Care (www.lucamp.org), and the Novo Nordisk Foundation Center for Basic Metabolic Research, which is an independent Research Center at the University of Copenhagen partially supported by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk). This work was supported by Ellen and Aage Andersen’s Foundation and The AP Møller Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Christiansen, S., Hertz, C., Ferrero-Miliani, L. et al. Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur J Hum Genet 24, 1797–1802 (2016). https://doi.org/10.1038/ejhg.2016.118
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2016.118
This article is cited by
-
Whole exome sequencing with a focus on cardiac disease-associated genes in families of sudden unexplained deaths in Yunnan, southwest of China
BMC Genomics (2023)
-
Genetics and genomics of arrhythmic risk: current and future strategies to prevent sudden cardiac death
Nature Reviews Cardiology (2021)
-
Postmortale molekulargenetische Untersuchungen (molekulare Autopsie) bei kardiovaskulären und bei ungeklärten Todesfällen
Der Kardiologe (2021)
-
Genetic investigations of 100 inherited cardiac disease-related genes in deceased individuals with schizophrenia
International Journal of Legal Medicine (2021)
-
Multiallelic rare variants support an oligogenic origin of sudden cardiac death in the young
Herz (2021)