Abstract
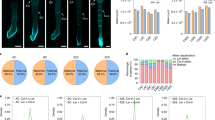
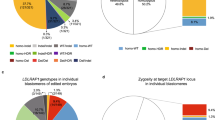
Gene expression from both parental genomes is required for completion of embryogenesis. Differential methylation of each parental genome has been observed in mouse and human preimplantation embryos. It is possible that these differences in methylation affect the level of gene transcripts from each parental genome in early developing embryos. The aim of this study was to investigate if there is a parent-specific pattern of BRCA1 expression in human embryos and to examine if this affects embryo development when the embryo carries a BRCA1 or BRCA2 pathogenic mutation. Differential parental expression of ACTB, SNRPN, H19 and BRCA1 was semi-quantitatively analysed by minisequencing in 95 human preimplantation embryos obtained from 15 couples undergoing preimplantation genetic diagnosis. BRCA1 was shown to be differentially expressed favouring the paternal transcript in early developing embryos. Methylation-specific PCR showed a variable methylation profile of BRCA1 promoter region at different stages of embryonic development. Embryos carrying paternally inherited BRCA1 or 2 pathogenic variants were shown to develop more slowly compared with the embryos with maternally inherited BRCA1 or 2 pathogenic mutations. This study suggests that differential demethylation of the parental genomes can influence the early development of preimplantation embryos. Expression of maternal and paternal genes is required for the completion of embryogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
McGrath J, Solter D : Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 1984; 37: 179–183.
Monk M, Boubelik M, Lehnert S : Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 1987; 99: 371–382.
Rougier N, Bourc'his D, Gomes D M et al: Chromosome methylation patterns during mammalian preimplantation development. Genes Dev 1998; 12: 2108–2113.
Dean W, Santos F, Stojkovic M et al: Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci USA 2001; 98: 13734–13738.
Swald J, Engemann S, Lane N et al: Active demethylation of the paternal genome in the mouse zygote. Curr Biol 2000; 10: 475–478.
Mayer W, Niveleau A, Walter J, Fundele R, Haaf T : Demethylation of the zygotic paternal genome. Nature 2000; 403: 501–502.
Beaujean N, Hartshorne G, Cavilla J et al: Non-conservation of mammalian preimplantation methylation dynamics. Curr Biol 2004; 14: R266–R267.
Santos F, Dean W : Epigenetic reprogramming during early development in mammals. Reproduction 2004; 127: 643–651.
Santos F, Hendrich B, Reik W, Dean W : Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 2002; 241: 172–182.
Howlett SK, Reik W : Methylation levels of maternal and paternal genomes during preimplantation development. Development 1991; 113: 119–127.
Omizawa S, Kobayashi H, Watanabe T et al: Dynamic stage-specific changes in imprinted differentially methylated regions during early mammalian development and prevalence of non-CpG methylation in oocytes. Development 2011; 138: 811–820.
Zhao MT, Rivera RM, Prather RS : Locus-specific DNA methylation reprogramming during early porcine embryogenesis. Biol Reprod 2013; 88: 48.
Paranjpe SS, Veenstra GJ : Establishing pluripotency in early development. Biochim Biophys Acta 2015; 1849: 626–636.
Magdinier F, D'estaing SG, Peinado C et al: Epigenetic marks at BRCA1 and p53 coding sequences in early human embryogenesis. Mol Hum Reprod 2002; 8: 630–635.
Muttukrishna S, Mcgarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P : Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG 2005; 112: 1384–1390.
Sahu B, Ozturk O, Deo N, Fordham K, Ranierri M, Serhal P : Response to controlled ovarian stimulation and oocyte quality in women with myotonic dystrophy type I. J Assist Reprod Genet 2008; 25: 1–5.
Bolton VN, Hawes SM, Taylor CT, Parsons JH : Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastocyst. J In Vitro Fert Embryo Transf 1989; 6: 30–35.
Esteller M, Silva JM, Dominguez G et al: Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst 2000; 92: 564–569.
Ibala-Romdhane S, Al-Khtib M, Khoueiry R, Blachere T, Guerin JF, Lefevre A : Analysis of H19 methylation in control and abnormal human embryos, sperm and oocytes. Eur J Hum Genet 2011; 19: 1138–1143.
Monk M, Salpekar A : Expression of imprinted genes in human preimplantation development. Mol Cell Endocrinol 2001; 183 (Suppl 1): S35–S40.
Khoueiry R, Ibala-Romdhane S, Al-Khtib M : Abnormal methylation of KCNQ1OT1 and differential methylation of H19 imprinting control regions in human ICSI embryos. Zygote 2012; 21: 129–138.
Huntriss J, Daniels R, Bolton V, Monk M : Imprinted expression of SNRPN in human preimplantation embryos. Am J Hum Genet 1998; 63: 1009–1014.
Lighten AD, Hardy K, Winston RM, Moore GE : IGF2 is parentally imprinted in human preimplantation embryos. Nat Genet 1997; 15: 122–123.
DeBaun MR, Niemitz EL, Feinberg AP : Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003; 72: 156–160.
Orstavik KH, Eiklid K, Van Der Hagen CB : Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet 2003; 72: 218–219.
Gicquel C, Gaston V, Mandelbaum J, Siffroi JP. Flahault A, Le Bouc Y : In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet 2003; 72: 1338–1341.
Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T : Aberrant DNA methylation of imprinted loci in superovulated oocytes. Hum Reprod 2007; 22: 26–35.
Orban TI, Olah E : Emerging roles of BRCA1 alternative splicing. Mol Pathol 2003; 56: 191–197.
Lixia M, Zhijian C, Chao S, Chaojiang G, Congyi Z : Alternative splicing of breast cancer associated gene BRCA1 from breast cancer cell line. J Biochem Mol Biol 2007; 40: 15–21.
Wilson CA, Payton MN, Elliott GS et al: Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta11b. Oncogene 1997; 14: 1–16.
Tammaro C, Raponi M, Wilson DI, Baralle D : BRCA1 exon 11 alternative splicing, multiple functions and the association with cancer. Biochem Soc Trans 2012; 40: 768–772.
Borel C, Ferreira PG, Santoni F et al: Biased allelic expression in human primary fibroblast single cells. Am J Hum Genet 2015; 96: 70–80.
Senst N, Llacuachaqui M, Lubinski J et al: Parental origin of mutation and the risk of breast cancer in a prospective study of women with a BRCA1 or BRCA2 mutation. Clin Genet 2012; 84: 43–46.
Ellberg C, Jernström H, Broberg P, Borg Å, Olsson H : Impact of a paternal origin of germline BRCA1/2 mutations on the age at breast and ovarian cancer diagnosis in a Southern Swedish cohort. Genes Chromosomes Cancer 2014; 54: 39–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
PT and SSB: substantial contributions to conception and design. PT, AD and PS: acquisition of data. PT and SSB: interpretation of data. PT: analysis and drafting the article. PT, AD, PS and SSB: revising it critically for important intellectual content, and final approval of the version to be published.
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Tulay, P., Doshi, A., Serhal, P. et al. Differential expression of parental alleles of BRCA1 in human preimplantation embryos. Eur J Hum Genet 25, 37–42 (2017). https://doi.org/10.1038/ejhg.2016.121
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2016.121
This article is cited by
-
Outcomes of BRCA pre-implantation genetic testing according to the parental mutation origin: a cohort study
Reproductive Biology and Endocrinology (2024)