Abstract
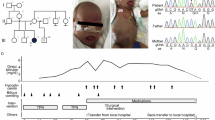
Beckwith–Wiedemann syndrome (BWS; OMIM #130650) is an overgrowth syndrome caused by different genetic or epigenetic alterations affecting imprinted regions on chromosome 11p15.5. Here we report a family with multiple offspring affected with BWS including giant omphalocoeles in which maternal transmission of a chromosomal rearrangement including an inversion and two deletions leads to hypomethylation of the imprint control region 2 (ICR2). As the deletion includes the promoter and 5′ part of the KCNQ1 gene, we suggest that transcription of this gene may be involved in establishing the maternal methylation imprint of the ICR2, which is located in intron 10 of KCNQ1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weksberg R, Shuman C, Beckwith JB : Beckwith–Wiedemann syndrome. Eur J Hum Genet 2010; 18: 8–14.
Eggermann T, Algar E, Lapunzina P et al: Clinical utility gene card for: Beckwith–Wiedemann Syndrome. Eur J Hum Genet 2014; 22: doi:10.1038/ejhg.2013.132.
Eggermann T, Netchine I, Temple IK et al: Congenital imprinting disorders: EUCID.net – a network to decipher their aetiology and to improve the diagnostic and clinical care. Clin Epigenet 2015; 7: 23.
Choufani S, Shuman C, Weksberg R : Beckwith–Wiedemann syndrome. Am J Med Genet C 2010; 154C: 343–354.
Choufani S, Shuman C, Weksberg R : Molecular findings in Beckwith–Wiedemann syndrome. Am J Med Genet C 2013; 163C: 131–140.
Begemann M, Spengler S, Gogiel M et al: Clinical significance of copy number variations in the 11p15.5 imprinting control regions: new cases and review of the literature. J Med Genet 2012; 49: 547–553.
Baskin B, Choufani S, Chen YA et al: High frequency of copy number variations (CNVs) in the chromosome 11p15 region in patients with Beckwith–Wiedemann syndrome. Hum Genet 2014; 133: 321–330.
Gurrieri F, Zollino M, Oliva A et al: Mild Beckwith–Wiedemann and severe long-QT syndrome due to deletion of the imprinting center 2 on chromosome 11p. Eur J Hum Genet 2013; 21: 11–969.
Niemitz EL, DeBaun MR, Fallon J et al: Microdeletion of LIT1 in familial Beckwith–Wiedemann syndrome. Am J Hum Genet 2004; 75: 844–849.
Algar E, Dagar V, Sebaj M, Pachter N : An 11p15 imprinting centre region 2 deletion in a family with Beckwith–Wiedemann syndrome provides insights into imprinting control at CDKN1C. PLoS One 2011; 6: e29034.
Zollino M, Orteschi D, Marangi G et al: A case of Beckwith–Wiedemann syndrome caused by a cryptic 11p15 deletion encompassing the centromeric imprinted domain of the BWS locus. J Med Genet 2010; 47: 429–432.
Demars J, Rossignol S, Netchine I et al: New insights into the pathogenesis of Beckwith–Wiedemann and Silver–Russell syndromes: contribution of small copy number variations to 11p15 imprinting defects. Hum Mutat 2011; 32: 1171–1182.
Kolarova J, Tangen I, Bens S et al: Array-based DNA methylation analysis in individuals with developmental delay/intellectual disability and normal molecular karyotype. Eur J Med Genet 2015; 58: 419–425.
Court F, Tayama C, Romanelli V et al: Genome-wide parent-of-origin DNA methylation analysis reveals the intricacies of human imprinting and suggests a germline methylation-independent mechanism of establishment. Genome Res 2014; 24: 554–569.
Wieczorek D, Newman WG, Wieland T et al: Compound heterozygosity of low-frequency promoter deletions and rare loss-of-function mutations in TXNL4A causes Burn–McKeown syndrome. Am J Hum Genet 2014; 95: 698–707.
Eggermann T, Heilsberg AK, Bens S et al: Additional molecular findings in 11p15-associated imprinting disorders: an urgent need for multi-locus testing. J Mol Med (Berl) 2014; 92: 769–777.
Cerrato F, De Crescenzo A, Riccio A : Looking for CDKN1C enhancers. Eur J Hum Genet 2014; 22: 442–443.
Neyroud N, Richard P, Vignier N et al: Genomic organization of the KCNQ1 K+ channel gene and identification of C-terminal mutations in the long-QT syndrome. Circ Res 1999; 84: 290–297.
Zhang P, Kerkela E, Skottman H et al: Distinct sets of developmentally regulated genes that are expressed by human oocytes and human embryonic stem cells. Fertil Steril 2007; 87: 677–690.
Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R : Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod 2010; 25: 957–968.
Yan Y, Frisen J, Lee MH, Massague J, Barbacid M : Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev 1997; 11: 973–983.
Zhang P, Liegeois NJ, Wong C et al: Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith–Wiedemann syndrome. Nature 1997; 387: 151–158.
Bonaldi A, Mazzeu JF, Costa SS et al: Microduplication of the ICR2 domain at chromosome 11p15 and familial Silver–Russell syndrome. Am J Med Genet A 2011; 155A: 2479–2483.
Chotalia M, Smallwood SA, Ruf N et al: Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev 2009; 23: 105–117.
Lewis MW, Brant JO, Kramer JM et al: Angelman syndrome imprinting center encodes a transcriptional promoter. Proc Natl Acad Sci USA 2015; 112: 6871–6875.
Smith EY, Futtner CR, Chamberlain SJ, Johnstone KA, Resnick JL : Transcription is required to establish maternal imprinting at the Prader–Willi syndrome and Angelman syndrome locus. PLoS Genet 2011; 7: e1002422.
John RM, Ainscough JF, Barton SC, Surani MA : Distant cis-elements regulate imprinted expression of the mouse p57(Kip2) (Cdkn1c) gene: implications for the human disorder, Beckwith—Wiedemann syndrome. Hum Mol Genet 2001; 10: 1601–1609.
Xue Z, Huang K, Cai C et al: Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013; 500: 593–597.
Yerushalmi GM, Salmon-Divon M, Yung Y et al: Characterization of the human cumulus cell transcriptome during final follicular maturation and ovulation. Mol Hum Reprod 2014; 20: 719–735.
Acknowledgements
We are grateful to the family for their participation in the study, Dagmar Wieczorek for initiating WGS, Nuria Brämswig for her help regarding the WGS data and Sabine Kaya, Melanie Heitmann and Christina Lich for expert technical assistance. This work was funded by the Bundesministerium für Bildung und Forschung (BMBF; Imprinting diseases, Grant No. 01GM1513A and D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Beygo, J., Joksic, I., Strom, T. et al. A maternal deletion upstream of the imprint control region 2 in 11p15 causes loss of methylation and familial Beckwith–Wiedemann syndrome. Eur J Hum Genet 24, 1280–1286 (2016). https://doi.org/10.1038/ejhg.2016.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2016.3
This article is cited by
-
Ongoing Challenges in the Diagnosis of 11p15.5-Associated Imprinting Disorders
Molecular Diagnosis & Therapy (2022)
-
Frequency of KCNQ1 variants causing loss of methylation of Imprinting Centre 2 in Beckwith-Wiedemann syndrome
Clinical Epigenetics (2020)
-
Common genetic variation in the Angelman syndrome imprinting centre affects the imprinting of chromosome 15
European Journal of Human Genetics (2020)
-
Genomic imprinting disorders: lessons on how genome, epigenome and environment interact
Nature Reviews Genetics (2019)
-
Disruption of KCNQ1 prevents methylation of the ICR2 and supports the hypothesis that its transcription is necessary for imprint establishment
European Journal of Human Genetics (2019)