Abstract
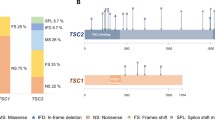
Structural brain malformations associated with Tuberous Sclerosis Complex (TSC) are related to the severity of the clinical symptoms and can be visualized by magnetic resonance imaging (MRI). Tuberous Sclerosis Complex is caused by inactivating TSC1 or TSC2 mutations. We investigated associations between TSC brain pathology and different inactivating TSC1 and TSC2 variants, and examined the potential prognostic value of subdivision of TSC2 variants based on their predicted effects on TSC2 expression. We performed genotype-phenotype associations of TSC-related brain pathology on a cohort of 64 children aged 1.4–17.9 years. Brain abnormalities were assessed using MRI. Individuals were grouped into those with an inactivating TSC1 variant and those with an inactivating TSC2 variant. The TSC2 group was subdivided into changes predicted to result in TSC2 protein expression (TSC2p) and changes predicted to prevent expression (TSC2x). The TSC2 group was associated with more and larger tubers, more radial migration lines, and more subependymal nodules than the TSC1 group. Subependymal nodules were also more likely to be calcified. Subdivision of the TSC2 group did not reveal additional, substantial differences, except for a larger number of tubers in the temporal lobe and a larger fraction of cystic tubers in the TSC2x subgroup. The severity of TSC-related brain pathology was related to the presence of an inactivating TSC2 variant. Although larger studies might find specific TSC2 variants that have prognostic value, in our cohort, subdivision of the TSC2 group did not lead to better prediction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nellist M, Janssen B, Brookcarter PT et al: Identification and characterization of the tuberous sclerosis gene on chromosome-16. Cell 1993; 75: 1305–1315.
van Slegtenhorst M, deHoogt R, Hermans C et al: Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997; 277: 805–808.
Gao XS, Zhang Y, Arrazola P et al: Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 2002; 4: 699–704.
Bissler JJ, Kingswood JC, Radzikowska E et al: Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 817–824.
Franz DN, Belousova E, Sparagana S et al: Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013; 381: 125–132.
Au KS, Williams AT, Roach ES et al: Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 2007; 9: 88–100.
Bolton PF, Clifford M, Tye C et al: Intellectual abilities in tuberous sclerosis complex: risk factors and correlates from the Tuberous Sclerosis 2000 Study. Psychol Med 2015; 45: 2321–2331.
Curatolo PM, Moavero R, Roberto D, Graziola F : Genotype/phenotype correlations in tuberous sclerosis complex. Semin Pediatr Neurol 2015; 4: 259–273.
Dabora SL, Jozwiak S, Franz DN et al: Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001; 68: 64–80.
Doherty C, Goh S, Poussaint TY, Erdag N, Thiele EA : Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol 2005; 20: 837–841.
Jansen FE, Braams O, Vincken KL et al: Overlapping neurologic and cognitive phenotypes in patients with TSC1 or TSC2 mutations. Neurology 2008; 70: 908–915.
Kothare SV, Singh K, Chalifoux JR et al: Severity of manifestations in tuberous sclerosis complex in relation to genotype. Epilepsia 2014; 55: 1025–1029.
Sancak O, Nellist M, Goedbloed M et al: Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype-phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur J Hum Genet 2005; 13: 731–741.
van Eeghen AM, Black ME, Pulsifer MB, Kwiatkowski DJ, Thiele EA : Genotype and cognitive phenotype of patients with tuberous sclerosis complex. Eur J Hum Genet 2012; 20: 510–515.
Wong HT, McCartney DL, Lewis JC, Sampson JR, Howe CJ, de Vries PJ : Intellectual ability in tuberous sclerosis complex correlates with predicted effects of mutations on TSC1 and TSC2 proteins. J Med Genet 2015; 52: 815–822.
Hoogeveen-Westerveld M, Wentink M, van den Heuvel D et al: Functional assessment of variants in the TSC1 and TSC2 genes identified in individuals with Tuberous Sclerosis Complex. Hum Mutat 2011; 32: 424–435.
Chantranupong L, Wolfson RL, Orozco JM et al: The sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 2014; 9: 1–8.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F : Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013; 8: 2281–2308.
Nellist M, Brouwer RWW, Kockx CEM et al: Targeted Next Generation Sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Med Genet 2015; 16: e10.
Nagy E, Maquat LE : A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 1998; 23: 198–199.
Bolton PF, Griffiths PD : Association of tuberous sclerosis of temporal lobes with autism and atypical autism. Lancet 1997; 349: 392–395.
Chu-Shore CJ, Major P, Montenegro M, Thiele E : Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology 2009; 72: 1165–1169.
Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA : Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 2011; 76: 981–987.
Michelozzi C, Di Leo G, Galli F et al: Subependymal nodules and giant cell tumours in tuberous sclerosis complex patients: prevalence on MRI in relation to gene mutation. Child Nerv Syst 2013; 29: 249–254.
Boronat S, Caruso P, Thiele EA : Absence of subependymal nodules in patients with tubers suggests possible neuroectodermal mosaicism in tuberous sclerosis complex. Dev Med Child Neurol 2014; 56: 1207–1211.
Kozlowski P, Roberts P, Dabora S et al: Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet 2007; 121: 389–400.
Tyburczy ME, Dies KA, Glass J et al: Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with no mutation identified by conventional testing. PLoS Genet 2015; 11: e1005637.
Verhoef S, Bakker L, Tempelaars AM et al: High rate of mosaicism in tuberous sclerosis complex. Am J Hum Genet 1999; 64: 1632–1637.
Lyczkowski DA, Conant KD, Pulsifer MB et al: Intrafamilial phenotypic variability in tuberous sclerosis complex. J Child Neurol 2007; 22: 1348–1355.
Crino PB, Aronica E, Baltuch G, Nathanson KL : Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology 2010; 74: 1716–1723.
Goorden SMI, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y : Cognitive deficits in Tsc1(+/−)mice in the absence of cerebral lesions and seizures. Ann Neurol 2007; 62: 648–655.
van Eeghen AM, Teran LO, Johnson J, Pulsifer MB, Thiele EA, Caruso P : The neuroanatomical phenotype of tuberous sclerosis complex: focus on radial migration lines. Neuroradiology 2013; 55: 1007–1014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R Swenker reports financial assistance from Novartis. MCY de Wit reports grants from Dutch Epilepsy Foundation, and grants and non-financial support from Novartis outside the submitted work; and the Erasmus MC received honoraria from Novartis for educational lectures presented by the author. The remaining authors declare no conflict of interest.
Additional information
Author contributions
IE Overwater contributed to study design, data collection, data analysis, data interpretation and writing of the report. R Swenker contributed to study design, experimental work, data collection, data analysis, data interpretation and writing of the report. EL van der Ende contributed to data collection, data analysis and writing of the report. KBM Hanemaayer contributed to data collection, data analysis and writing of the report. M Hoogeveen-Westerveld contributed to experimental work, data collection, data analysis and data interpretation, and writing of the report. AM van Eeghen contributed to study design, and writing of the report. MH Lequin contributed to study design, data collection and writing of the report. AMW van den Ouweland contributed to data collection, and writing of the report. HA Moll contributed to study design, data interpretation and writing of the report. M Nellist contributed to study design, experimental work, data collection, data analysis, data interpretation and writing of the report. MCY de Wit contributed to study design, data collection, data analysis, data interpretation and writing of the report.
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Overwater, I., Swenker, R., van der Ende, E. et al. Genotype and brain pathology phenotype in children with tuberous sclerosis complex. Eur J Hum Genet 24, 1688–1695 (2016). https://doi.org/10.1038/ejhg.2016.85
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2016.85
This article is cited by
-
A case report of severe tuberous sclerosis complex detected in utero and linked to a novel duplication in the TSC2 gene
BMC Neurology (2020)
-
Radiobiological Characterization of Tuberous Sclerosis: a Delay in the Nucleo-Shuttling of ATM May Be Responsible for Radiosensitivity
Molecular Neurobiology (2018)
-
The origin and natural history of autism spectrum disorders
Nature Neuroscience (2016)