Abstract
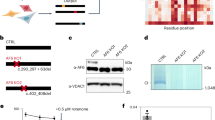
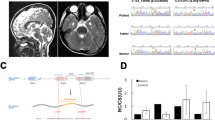
Mitochondrial respiratory chain complex I consists of 44 different subunits and can be subgrouped into three functional modules: the Q-, the P- and the N-module. NDUFAF4 (C6ORF66) is an assembly factor of complex I that associates with assembly intermediates of the Q-module. Via exome sequencing, we identified a homozygous missense variant in a complex I-deficient patient with Leigh syndrome. Supercomplex analysis in patient fibroblasts revealed specifically altered stoichiometry. Detailed assembly analysis of complex I, indicative of all of its assembly routes, showed an accumulation of parts of the P- and the N-module but not the Q-module. Lentiviral complementation of patient fibroblasts with wild-type NDUFAF4 rescued complex I deficiency and the assembly defect, confirming the causal role of the variant. Our report on the second family affected by an NDUFAF4 variant further characterizes the phenotypic spectrum and sheds light into the role of NDUFAF4 in mitochondrial complex I biogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Guerrero-Castillo S, Baertling F, Kownatzki D et al: The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab 2017; 25: 128–139.
Saada A, Edvardson S, Rapoport M et al: C6ORF66 is an assembly factor of mitochondrial complex I. Am J Hum Genet 2008; 82: 32–38.
Schwarz JM, Cooper DN, Schuelke M, Seelow D : MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014; 11: 361–362.
Adzhubei IA, Schmidt S, Peshkin L et al: A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Kumar P, Henikoff S, Ng PC : Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081.
Saada A, Vogel RO, Hoefs SJ et al: Mutations in NDUFAF3 (C3ORF60), encoding an NDUFAF4 (C6ORF66)-interacting complex I assembly protein, cause fatal neonatal mitochondrial disease. Am J Hum Genet 2009; 84: 718–727.
Baertling F, Sanchez-Caballero L, Timal S et al: Mutations in mitochondrial complex I assembly factor NDUFAF3 cause Leigh syndrome. Mol Genet Metab 2016; 120: 243–246.
Baertling F, Rodenburg RJ, Schaper J et al: A guide to diagnosis and treatment of Leigh syndrome. J Neurol Neurosurg Psychiatry 2014; 85: 257–265.
Saada A, Edvardson S, Shaag A et al: Combined OXPHOS complex I and IV defect, due to mutated complex I assembly factor C20ORF7. J Inherit Metab Dis 2012; 35: 125–131.
Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U : Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem 2004; 279: 36349–36353.
Marcus D, Lichtenstein M, Saada A, Lorberboum-Galski H : Replacement of the C6ORF66 assembly factor (NDUFAF4) restores complex I activity in patient cells. Mol Med 2013; 19: 124–134.
Zurita Rendon O, Shoubridge EA : Early complex I assembly defects result in rapid turnover of the ND1 subunit. Hum Mol Genet 2012; 21: 3815–3824.
Hornig-Do HT, Tatsuta T, Buckermann A et al: Nonsense mutations in the COX1 subunit impair the stability of respiratory chain complexes rather than their assembly. EMBO J 2012; 31: 1293–1307.
Acknowledgements
We thank the mitochondrial diagnostics group (muscle lab, cell culture lab and DNA lab) of the Radboud Center for Mitochondrial Medicine (RCMM) at the Translational Metabolic Laboratory, Radboud UMC for excellent technical assistance. We would like to acknowledge the Genome Technology Center at the Radboud UMC and BGI Copenhagen for providing the exome sequencing service. This project was funded by the European Commission (FP7-PEOPLE-ITN. GA. 317433). Part of this work was financed by a grant obtained from the United Mitochondrial Disease Foundation (UMDF). FB was supported by a fellowship of the German Research Foundation/Deutsche Forschungsgemeinschaft (BA 5758/1-1).
Informed consent
Informed consent for diagnostic and research studies was obtained for all subjects in accordance with the Declaration of Helsinki and following the regulations of the local medical ethics committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Baertling, F., Sánchez-Caballero, L., van den Brand, M. et al. NDUFAF4 variants are associated with Leigh syndrome and cause a specific mitochondrial complex I assembly defect. Eur J Hum Genet 25, 1273–1277 (2017). https://doi.org/10.1038/ejhg.2017.133
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2017.133
This article is cited by
-
Pathogenic morphological signatures of perturbations in mitochondrial-related genes revealed by pooled imaging assay
npj Imaging (2025)
-
Multiomics analysis identifies oxidative phosphorylation as a cancer vulnerability arising from myristoylation inhibition
Journal of Translational Medicine (2024)
-
Protein lipidation in cancer: mechanisms, dysregulation and emerging drug targets
Nature Reviews Cancer (2024)
-
The host cellular protein Ndufaf4 interacts with the vesicular stomatitis virus M protein and affects viral propagation
Virus Genes (2021)
-
Molecular basis of Leigh syndrome: a current look
Orphanet Journal of Rare Diseases (2020)