Abstract
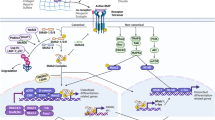
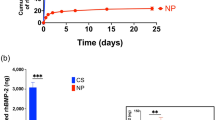
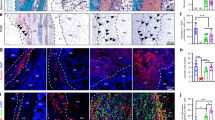
Thoracic ossification of the ligamentum flavum (TOLF)is a common cause of thoracic spinal canal stenosis and has been reported almost exclusively in East Asian countries. In this study, we established a relationship between bone morphogenic protein 2 (BMP-2) and TOLF. We divided patients into two groups according to severity of ossification and identified susceptible loci through exome sequencing. We identified 39 novel likely pathogenic variants in 29 genes in the transforming growth factor-beta (TGF-β) superfamily or TGF-β/BMPs signaling pathway, including two missense variants in BMP-2 (NM_001200.3) exon region, c.460C>G:p.(R154G) and c.584G>T:p.(R195M). Further Sanger sequencing and genotyping suggested the variants were only found in patients with long regional OLF. Bioinformatic assays predicted the two BMP-2 variants to cause significant alterations to gene and protein expression. Functional assays showed upregulation of BMP-2 expression, increased osteogenic marker expression, and enhanced osteogenic differentiation. Collectively, these results suggest a genetic contribution to the pathogenesis of TOLF, particularly in patients with long segment disease, and that nucleotide substitutions associated with increased BMP-2 expression may be involved in TOLF pathogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Feng FB, Sun CG, Chen ZQ : Progress on clinical characteristics and identification of location of thoracic ossification of the ligamentum flavum. Orthop Surg 2015; 7: 87–96.
Hou X, Sun C, Liu X et al: Clinical features of thoracic spinal stenosis-associated myelopathy: a retrospective analysis of 427 cases. Clin Spine Surg 2016; 29: 86–89.
Lang N, Yuan HS, Wang HL et al: Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur Spine J 2013; 22: 857–862.
Kong Q, Ma X, Li F et al: COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2007; 32: 2834–2838.
Liu Y, Zhao Y, Chen Y, Shi G, Yuan W : RUNX2 polymorphisms associated with OPLL and OLF in the Han population. Clin Orthop Relat Res 2010; 468: 3333–3341.
Karasugi T, Nakajima M, Ikari K et al: A genome-wide sib-pair linkage analysis of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Metab 2013; 31: 136–143.
Wilson JR, Patel AA, Brodt ED, Dettori JR, Brodke DS, Fehlings MG : Genetics and heritability of cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: results of a systematic review. Spine (Phila Pa 1976) 2013; 38: S123–S146.
Horikoshi T, Maeda K, Kawaguchi Y et al: A large-scale genetic association study of ossification of the posterior longitudinal ligament of the spine. Hum Genet 2006; 119: 611–616.
Tanaka T, Ikari K, Furushima K et al: Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet 2003; 73: 812–822.
Miyasaka K, Kaneda K, Sato S et al: Myelopathy due to ossification or calcification of the ligamentum flavum: radiologic and histologic evaluations. AJNR Am J Neuroradiol 1983; 4: 629–632.
Chen G, Deng C, Li YP : TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012; 8: 272–288.
Tsumaki N, Yoshikawa H : The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev 2005; 16: 279–285.
Hou XF, Fan DW, Sun CG, Chen ZQ : Recombinant human bone morphogenetic protein-2-induced ossification of the ligamentum flavum in rats and the associated global modification of histone H3. J Neurosurg Spine 2014; 21: 334–341.
Kamiya M, Harada A, Mizuno M, Iwata H, Yamada Y : Association between a polymorphism of the transforming growth factor-beta1 gene and genetic susceptibility to ossification of the posterior longitudinal ligament in Japanese patients. Spine (Phila Pa 1976) 2001; 26: 1264–1266, discussion 1266–1267.
Jekarl DW, Paek CM, An YJ et al: TGFBR2 gene polymorphism is associated with ossification of the posterior longitudinal ligament. J Clin Neurosci 2013; 20: 453–456.
Wang H, Yang ZH, Liu DM, Wang L, Meng XL, Tian BP : Association between two polymorphisms of the bone morpho-genetic protein-2 gene with genetic susceptibility to ossification of the posterior longitudinal ligament of the cervical spine and its severity. Chin Med J (Engl) 2008; 121: 1806–1810.
Furushima K, Shimo-Onoda K, Maeda S et al: Large-scale screening for candidate genes of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res 2002; 17: 128–137.
Ren Y, Liu ZZ, Feng J et al: Association of a BMP9 haplotype with ossification of the posterior longitudinal ligament (OPLL) in a Chinese population. PLoS One 2012; 7: e40587.
Yan L, Chang Z, Liu Y, Li YB, He BR, Hao DJ : A single nucleotide polymorphism in the human bone morphogenetic protein-2 gene (109T>G) affects the Smad signaling pathway and the predisposition to ossification of the posterior longitudinal ligament of the spine. Chin Med J (Engl) 2013; 126: 1112–1118.
Wang H, Liu D, Yang Z et al: Association of bone morphogenetic protein-2 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the spine and its severity in Chinese patients. Eur Spine J 2008; 17: 956–964.
Chen ZQ, Sun CG : Clinical guideline for treatment of symptomatic thoracic spinal stenosis. Orthop Surg 2015; 7: 208–212.
Li H, Durbin R : Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25: 1754–1760.
DePristo MA, Banks E, Poplin R et al: A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011; 43: 491–498.
Wang K, Li M, Hakonarson H : ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38: e164.
Qu X, Chen Z, Fan D, Sun C, Zeng Y : MiR-132-3p regulates the osteogenic differentiation of thoracic ligamentum flavum cells by inhibiting multiple osteogenesis-related genes. Int J Mol Sci 2016; 17.
Qu X, Chen Z, Fan D et al: Notch signaling pathways in human thoracic ossification of the ligamentum flavum. J Orthop Res 2016; 34: 1481–1491.
Moon BJ, Kuh SU, Kim S, Kim KS, Cho YE, Chin DK : Prevalence, distribution, and significance of incidental thoracic ossification of the ligamentum flavum in Korean patients with back or leg pain: MR-Based Cross Sectional Study. J Korean Neurosurg Soc 2015; 58: 112–118.
Mori K, Kasahara T, Mimura T et al: Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese: results of CT-based cross-sectional study. Spine (Phila Pa 1976) 2013; 38: E1216–E1222.
Kudo H, Furukawa K, Yokoyama T et al: Genetic differences in the osteogenic differentiation potency according to the classification of ossification of the posterior longitudinal ligament of the cervical spine. Spine (Phila Pa 1976) 2011; 36: 951–957.
Shi M, Zhu J, Wang R et al: Latent TGF-beta structure and activation. Nature 2011; 474: 343–349.
Moustakas A, Heldin CH : The regulation of TGFbeta signal transduction. Development 2009; 136: 3699–3714.
Kon T, Yamazaki M, Tagawa M et al: Bone morphogenetic protein-2 stimulates differentiation of cultured spinal ligament cells from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 1997; 60: 291–296.
Yang IH, Kim H, Kwon UH et al: De novo osteogenesis from human ligamentum flavum by adenovirus-mediated bone morphogenetic protein-2 gene transfer. Spine (Phila Pa 1976) 2005; 30: 2749–2754.
Hoshi K, Amizuka N, Sakou T, Kurokawa T, Ozawa H : Fibroblasts of spinal ligaments pathologically differentiate into chondrocytes induced by recombinant human bone morphogenetic protein-2: morphological examinations for ossification of spinal ligaments. Bone 1997; 21: 155–162.
Tanaka H, Nagai E, Murata H et al: Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology (Oxford) 2001; 40: 1163–1168.
Moon SH, Park SR, Kim H et al: Biologic modification of ligamentum flavum cells by marker gene transfer and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2004; 29: 960–965.
Hayashi K, Ishidou Y, Yonemori K et al: Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone 1997; 21: 23–30.
Yokosuka K, Park JS, Jimbo K et al: Immunohistochemical demonstration of advanced glycation end products and the effects of advanced glycation end products in ossified ligament tissues in vitro. Spine (Phila Pa 1976) 2007; 32: E337–E339.
Sato R, Uchida K, Kobayashi S et al: Ossification of the posterior longitudinal ligament of the cervical spine: histopathological findings around the calcification and ossification front. J Neurosurg Spine 2007; 7: 174–183.
Yayama T, Uchida K, Kobayashi S et al: Thoracic ossification of the human ligamentum flavum: histopathological and immunohistochemical findings around the ossified lesion. J Neurosurg Spine 2007; 7: 184–193.
Li JM, Zhang Y, Ren Y et al: Uniaxial cyclic stretch promotes osteogenic differentiation and synthesis of BMP2 in the C3H10T1/2 cells with BMP2 gene variant of rs2273073 (T/G). PLoS One 2014; 9: e106598.
Li J, Yan M, Wang Z et al: Effects of canonical NF-kappaB signaling pathway on the proliferation and odonto/osteogenic differentiation of human stem cells from apical papilla. Biomed Res Int 2014; 2014: 319651.
Acknowledgements
We are grateful to all the patients for providing blood samples and specimens. We acknowledge the assistance of Beijing Novogene Bioinformatics Technology Co., Ltd (http://www.novogene.com) with exon sequencing and Beijing Ubiolab Technology Co., Ltd (http://www.ubiolab.com/) with Sanger sequencing. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81272031, 81071505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Qu, X., Chen, Z., Fan, D. et al. Two novel BMP-2 variants identified in patients with thoracic ossification of the ligamentum flavum. Eur J Hum Genet 25, 565–571 (2017). https://doi.org/10.1038/ejhg.2017.2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2017.2
This article is cited by
-
METTL3 regulated by histone lactylation promotes ossification of the ligamentum flavum by enhancing the m6A methylation of BMP2
Molecular Medicine (2025)
-
Biological mechanisms underlying the ossification of ligamentum flavum and potential therapeutic targets derived from current research
European Spine Journal (2025)
-
Significance of body mass index on thoracic ossification of the ligamentum flavum in Chinese population
European Spine Journal (2022)
-
Disruption of the mouse Bmal1 locus promotes heterotopic ossification with aging via TGF-beta/BMP signaling
Journal of Bone and Mineral Metabolism (2022)
-
Association analysis and functional study of COL6A1 single nucleotide polymorphisms in thoracic ossification of the ligamentum flavum in the Chinese Han population
European Spine Journal (2021)