Abstract
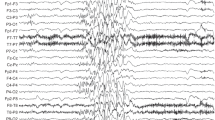
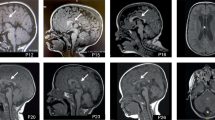
For a large number of individuals with intellectual disability (ID), the molecular basis of the disorder is still unknown. However, whole-exome sequencing (WES) is providing more and more insights into the genetic landscape of ID. In the present study, we performed trio-based WES in 311 patients with unsolved ID and additional clinical features, and identified homozygous CPLX1 variants in three patients with ID from two unrelated families. All displayed marked developmental delay and migrating myoclonic epilepsy, and one showed a cerebellar cleft in addition. The encoded protein, complexin 1, is crucially involved in neuronal synaptic regulation, and homozygous Cplx1 knockout mice have the earliest known onset of ataxia seen in a mouse model. Recently, a homozygous truncating variant in CPLX1 was suggested to be causative for migrating epilepsy and structural brain abnormalities. ID was not reported although it cannot be completely ruled out. However, the currently limited knowledge on CPLX1 suggests that loss of complexin 1 function may lead to a complex but variable clinical phenotype, and our findings encourage further investigations of CPLX1 in patients with ID, developmental delay and myoclonic epilepsy to unravel the phenotypic spectrum of carriers of CPLX1 variants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Trimbuch T, Rosenmund C : Should I stop or should I go? The role of complexin in neurotransmitter release. Nat Rev Neurosci 2016; 17: 118–125.
Snead D, Wragg RT, Dittman JS, Eliezer D : Membrane curvature sensing by the C-terminal domain of complexin. Nat Commun 2014; 5: 4955.
Brose N : Altered complexin expression in psychiatric and neurological disorders: cause or consequence? Mol Cells 2008; 25: 7–19.
Drew CJ, Kyd RJ, Morton AJ : Complexin 1 knockout mice exhibit marked deficits in social behaviours but appear to be cognitively normal. Hum Mol Genet 2007; 16: 2288–2305.
Kielar C, Sawiak SJ, Navarro Negredo P, Tse DHY, Morton AJ : Tensor-based morphometry and stereology reveal brain pathology in the complexin1 knockout mouse. PLoS One 2012; 7: e32636.
Karaca E, Harel T, Pehlivan D et al: Genes that affect brain structure and function identified by rare variant analyses of Mendelian neurologic disease. Neuron 2015; 88: 499–513.
Kuechler A, Willemsen MH, Albrecht B et al: De novo mutations in beta-catenin (CTNNB1) appear to be a frequent cause of intellectual disability: expanding the mutational and clinical spectrum. Hum Genet 2014; 134: 97–109.
McTague A, Howell KB, Cross JH, Kurian MA, Scheffer IE : The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol 2015; 15: 304–316.
Glynn D, Drew CJ, Reim K, Brose N, Morton AJ : Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum Mol Genet 2005; 14: 2369–2385.
Glynn D, Sizemore RJ, Morton AJ : Early motor development is abnormal in complexin 1 knockout mice. Neurobiol Dis 2007; 25: 483–495.
Chang S, Reim K, Pedersen M, Neher E, Brose N, Taschenberger H : Complexin stabilizes newly primed synaptic vesicles and prevents their premature fusion at the mouse calyx of held synapse. J Neurosci 2015; 35: 8272–8290.
Bosemani T, Poretti A : Cerebellar disruptions and neurodevelopmental disabilities. Semin Fetal Neonatal Med 2016; 21: 339–348.
Acknowledgements
We are grateful to the families for participating in this study. We thank Sabine Kaya and Daniela Falkenstein for excellent technical assistance. This work was supported in part by the German Ministry of Research and Education (grant numbers 01GS08164, 01GS08167, 01GS08163, German Mental Retardation Network/MRNET) as part of the National Genome Research Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Redler, S., Strom, T., Wieland, T. et al. Variants in CPLX1 in two families with autosomal-recessive severe infantile myoclonic epilepsy and ID. Eur J Hum Genet 25, 889–893 (2017). https://doi.org/10.1038/ejhg.2017.52
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ejhg.2017.52
This article is cited by
-
Spotlight on mechanism of sudden unexpected death in epilepsy in Dravet syndrome
Translational Psychiatry (2025)
-
Developmental and epileptic encephalopathies
Nature Reviews Disease Primers (2024)
-
Mechanism of Antidepressant Action of (2R,6R)-6-Hydroxynorketamine (HNK) and Its Compounds: Insights from Proteomic Analysis
Molecular Neurobiology (2024)
-
GWAS meta-analysis reveals key risk loci in essential tremor pathogenesis
Communications Biology (2024)
-
Generation of iPSC lines (KAIMRCi003A, KAIMRCi003B) from a Saudi patient with Dravet syndrome carrying homozygous mutation in the CPLX1 gene and heterozygous mutation in SCN9A
Human Cell (2023)