Abstract
Background
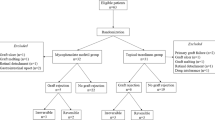
The purpose of this prospective, randomised, multicentre study was to prove the efficacy and safety of mycophenolate mofetil (MMF) in preventing graft rejection and in improving clear graft survival following high-risk keratoplasty.
Methods
In all, 98 of 140 scheduled patients were included in this study (57 MMF, 41 control). Recruitment was stopped prematurely due to a statistically significant result. The patients in the MMF group received MMF orally 2 × 1 g daily for 6 months. All of the patients received fluocortolone 1 mg/kg/day tapered over 3 weeks and topical prednisolone acetate 5 × /day tapered over 5 months. Main criteria were immune reaction-free and clear graft survival, and the occurrence of side effects.
Results
The mean follow-up time was 34.9±16.3 (mean±SD) months. Eleven patients withdrew from the study (nine patients due to protocol deviation, two because of side effects). Six reversible and two irreversible graft rejections occurred in the MMF group, and five reversible and seven irreversible rejections in the control group. The Kaplan–Meier analysis revealed an immune reaction-free graft survival after the mean follow-up time of 83% in the MMF group and 64.5% in the control group (P=0.044). Graft failure occurred in 10 MMF-treated patients (two due to rejection) and in nine patients in the control group (seven due to rejection). A total of 36 of 57 MMF-treated patients experienced mostly reversible adverse events.
Conclusions
Systemic immunosuppression with MMF over 6 months is relatively well tolerated and improves rejection-free graft survival following high-risk keratoplasty statistically significant, even in the long run.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hill JC . Systemic cyclosporine in high-risk keratoplasty. Short- vs long-term therapy. Ophthalmology 1994; 101: 128–133.
Reinhard T, Hutmacher M, Sundmacher R . [Acute and chronic immune reactions after penetrating keratoplasty with normal immune risk]. Klin Monatsbl Augenheilkd 1997; 210: 139–143.
Sundmacher R . [Allograft rejection reactions after keratoplasty (author's transl)]. Klin Monatsbl Augenheilkd 1977; 171: 705–722.
Birnbaum F, Böhringer D, Sokolovska Y, Sundmacher R, Reinhard T . Immunosuppression with cyclosporine a and mycophenolate mofetil after penetrating high-risk keratoplasty: a retrospective study. Transplantation 2005; 79: 964–968.
Birnbaum F, Reis A, Böhringer D, Sokolowska Y, Mayer K, Voiculescu A et al. An open prospective pilot study on the use of rapamycin after penetrating high-risk keratoplasty. Transplantation 2006; 81: 767–772.
Reinhard T, Sundmacher R, Godehardt E, Heering P . [Preventive systemic cyclosporin A after keratoplasty at increased risk for immune reactions as the only elevated risk factor]. Ophthalmologe 1997; 94: 496–500.
Reinhard T, Reis A, Böhringer D, Malinowski M, Voiculescu A, Heering P et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol 2001; 239: 367–372.
Reinhard T, Mayweg S, Sokolovska Y, Seitz B, Mittelviefhaus H, Engelmann K et al. Systemic mycophenolate mofetil avoids immune reactions in penetrating high-risk keratoplasty: preliminary results of an ongoing prospectively randomized multicentre study. Transpl Int 2005; 18: 703–708.
Allison AC, Eugui EM . Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000; 47: 85–118.
Allison AC, Eugui EM . Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation 2005; 80: S181–S190.
Morris RE, Hoyt EG, Murphy MP, Eugui EM, Allison AC . Mycophenolic acid morpholinoethylester (RS-61443) is a new immunosuppressant that prevents and halts heart allograft rejection by selective inhibition of T- and B-cell purine synthesis. Transplant Proc 1990; 22: 1659–1662.
Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995; 345: 1321–1325.
Mycophenolate mofetil in renal transplantation: 3-year results from the placebo-controlled trial. European Mycophenolate Mofetil Cooperative Study Group. Transplantation 1999; 68: 391–396.
A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996; 61: 1029–1037.
Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C . Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation 1997; 63: 39–47.
Rose ML, Smith J, Dureau G, Keogh A, Kobashigowa J . Mycophenolate mofetil decreases antibody production after cardiac transplantation. J Heart Lung Transplant 2002; 21: 282–285.
Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl 2001; 7: 442–450.
Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 1998; 66: 507–515.
Reis A, Reinhard T, Sundmacher R, Braunstein S, Godehardt E . Mycophenolate mofetil and FK506: two novel immunosuppressants in murine corneal transplantation. Transplant Proc 1998; 30: 4344–4347.
Reis A, Reinhard T, Sundmacher R, Braunstein C, Godehardt E . Effect of mycophenolate mofetil, cyclosporin A, and both in combination in a murine corneal graft rejection model. Br J Ophthalmol 1998; 82: 700–703.
Sundmacher R, Reinhard T . Central corneolimbal transplantation under systemic ciclosporin A cover for severe limbal stem cell insufficiency. Graefes Arch Clin Exp Ophthalmol 1996; 234 (Suppl 1): S122–S125.
Reinhard T, Sundmacher R . [Perforating keratoplasty in endogenous eczema. An indication for systemic cyclosporin A—a retrospective study of 18 patients]. Klin Monatsbl Augenheilkd 1992; 201: 159–163.
Sundmacher R, Stefansson A, Mackensen G . [Postoperative course after keratoplasty]. Fortschr Ophthalmol 1983; 80: 224–227.
Hoffmann F . [Suture technique for perforating keratoplasty (author′s transl)]. Klin Monatsbl Augenheilkd 1976; 169: 584–590.
Alldredge OC, Krachmer JH . Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol 1981; 99: 599–604.
Pleyer U, Weidle EG, Lisch W, Steuhl KP, Mohrle C, Richter U et al. [Clinical types of immunologic transplant reactions following perforating keratoplasty]. Fortschr Ophthalmol 1990; 87: 14–19.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Girard LJ, Esnaolo N, Rao R . Allograft rejection after penetrating keratoplasty for keratoconus. Ophthalmic Surg 1993; 24: 40–43.
Tompson RW, Price MO, Bowers PJ, Price FW . Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003; 110: 1396–1402.
Hill JC . The use of cyclosporine in high-risk keratoplasty. Am J Ophthalmol 1989; 107: 506–510.
Reinhard T, Sundmacher R, Heering P . Systemic ciclosporin A in high-risk keratoplasties. Graefes Arch Clin Exp Ophthalmol 1996; 234 (Suppl 1): S115–S121.
Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H et al. Mycophenolate mofetil vs cyclosporin A in high risk keratoplasty patients: a prospectively randomised clinical trial. Br J Ophthalmol 1999; 83: 1268–1271.
Sundmacher R, Reinhard T, Heering P . Six years' experience with systemic cyclosporin A prophylaxis in high-risk perforating keratoplasty patients. A retrospective study. Ger J Ophthalmol 1992; 1: 432–436.
Reinhard T, Reis A, Kutkuhn B, Voiculescu A, Sundmacher R . [Mycophenolate mofetil after penetrating high risk keratoplasty. A pilot study]. Klin Monatsbl Augenheilkd 1999; 215: 201–202.
Boots JM, Christiaans MH, van Hooff JP . Effect of immunosuppressive agents on long-term survival of renal transplant recipients: focus on the cardiovascular risk. Drugs 2004; 64: 2047–2073.
Lake JR, David KM, Steffen BJ, Chu AH, Gordon RD, Wiesner RH . Addition of MMF to dual immunosuppression does not increase the risk of malignant short-term death after liver transplantation. Am J Transplant 2005; 5: 2961–2967.
Robson R, Cecka JM, Opelz G, Budde M, Sacks S . Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 2005; 5: 2954–2960.
Acknowledgements
We thank Roche Pharma AG, Grenzach-Wyhlen, Germany, for financial support of the study.
Further participating centres: University Eye Hospital Düsseldorf, Germany; University Eye Hospital, Erlangen, Germany; University Eye Hospital Hamburg, Germany; University Eye Hospital München, Germany; University Eye Hospital Münster, Germany; University Eye Hospital Halle, Germany; University Eye Hospital Homburg/Saar, Germany; University Eye Hospital Essen, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented as free paper during the annual convention of the German Ophthalmological Society (DOG) in Berlin, 21–24 September 2006
Study registration number: NCT00411515; and ZKS UKF000727 (www.uniklinik-freiburg.de/zks/live/uklregister/Oeffentlich.html)
Rights and permissions
About this article
Cite this article
Birnbaum, F., Mayweg, S., Reis, A. et al. Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term results of a prospective, randomised, multicentre study. Eye 23, 2063–2070 (2009). https://doi.org/10.1038/eye.2008.402
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2008.402
Keywords
This article is cited by
-
Immunosuppressive Therapy for High-Risk Corneal Transplant
Current Ophthalmology Reports (2022)
-
Topical 0.03% tacrolimus versus systemic mycophenolate mofetil as adjuncts to systemic corticosteroids for preventing graft rejection after repeat keratoplasty: one-year results of a randomized clinical trial
Eye (2021)
-
Mycophenolate mofetil
Reactions Weekly (2018)
-
Twelve-year follow-up of penetrating keratoplasty
Japanese Journal of Ophthalmology (2017)
-
Systemic immunosuppression with mycophenolate mofetil to prevent corneal graft rejection after high-risk penetrating keratoplasty: a 2-year follow-up study
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)