Abstract
Purpose
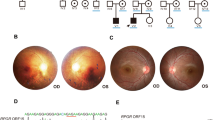
To document the progression of disease in male and female members of a previously described family with X-linked dominant retinitis pigmentosa (RP) caused by a de novoinsertion after nucleotide 173 in exon ORF15 of RPGR.
Methods
The clinical records of 19 members of family UTAD054 were reviewed. Their evaluations consisted of confirmation of family history, standardised electroretinograms (ERGs), Goldmann visual fields, and periodic ophthalmological examinations over a 23-year period.
Results
Male members of family UTAD054 had non-recordable to barely recordable ERGs from early childhood. The males showed contracted central fields and developed more severe retinopathy than the females. The female members showed a disease onset delayed to teenage years, recordable but diminishing photopic and scotopic ERG amplitudes in a cone-rod pattern, progressive loss and often asymmetric visual fields, and diffuse atrophic retinopathy with fewer pigment deposits compared with males.
Conclusions
This insertion mutation in the RPGRexon ORF15 is associated with a RP phenotype that severely affects males early and females by 30 years of age, and is highly penetrant in female members. Families with dominant-acting RPGRmutations may be mistaken to have an autosomal mode of inheritance resulting in an incorrect prediction of recurrence risk and prognosis. Broader recognition of X-linked RP forms with dominant inheritance is necessary to facilitate appropriate counselling of these patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Heckenlively JR . Retinitis Pigmentosa. Lippincott: Philadelphia, 1988.
Wang DY, Chan WM, Tam PO, Chiang SW, Lam DS, Chong KK et al. Genetic markers for retinitis pigmentosa. Hong Kong Med J 2005; 11 (4): 281–288.
Breuer DK, Yashar BM, Filippova E, Hiriyanna S, Lyons RH, Mears AJ et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet 2002; 70 (6): 1545–1554.
Sharon D, Sandberg MA, Rabe VW, Stillberger M, Dryja TP, Berson EL . RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am J Hum Genet 2003; 73 (5): 1131–1146.
Pelletier V, Jambou M, Delphin N, Zinovieva E, Stum M, Gigarel N et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: genotype-phenotype correlations and impact on genetic counseling. Hum Mutat 2007; 28 (1): 81–91.
Heckenlively JR, Rosales T, Martin D . Optic nerve changes in dominant cone-rod dystrophy. Doc Ophthal Proc Series 1981; 27: 183–192.
McGuire RE, Sullivan LS, Blanton SH, Church MW, Heckenlively JR, Daiger SP . X-linked dominant cone-rod degeneration: linkage mapping of a new locus for retinitis pigmentosa (RP 15) to Xp22.13-p22.11. Am J Hum Genet 1995; 57 (1): 87–94.
Mears AJ, Hiriyanna S, Vervoort R, Yashar B, Gieser L, Fahrner S et al. Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am J Hum Genet 2000; 67 (4): 1000–1003.
Al-Maskari A, O′Grady A, Pal B, McKibbin M . Phenotypic progression in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Eye 2009; 23 (3): 519–521.
Banin E, Mizrahi-Meissonnier L, Neis R, Silverstein S, Magyar I, Abeliovich D et al. A non-ancestral RPGR missense mutation in families with either recessive or semi-dominant X-linked retinitis pigmentosa. Am J Med Genet A 2007; 143A (11): 1150–1158.
Neidhardt J, Glaus E, Lorenz B, Netzer C, Li Y, Schambeck M et al. Identification of novel mutations in X-linked retinitis pigmentosa families and implications for diagnostic testing. Mol Vis 2008; 14: 1081–1093.
Rozet JM, Perrault I, Gigarel N, Souied E, Ghazi I, Gerber S et al. Dominant X linked retinitis pigmentosa is frequently accounted for by truncating mutations in exon ORF15 of the RPGR gene. J Med Genet 2002; 39 (4): 284–285.
Marmor M, Aguirre G, Arden G, Berson E, Birch D, Boughman J et al. Retinitis pigmentosa: a symposium on terminology and methods of examination. Ophthalmology 1983; 90: 126–131.
Fishman GA, Weinberg AB, McMahon TT . X-linked recessive retinitis pigmentosa. Clinical characteristics of carriers. Arch Ophthalmol 1986; 104 (9): 1329–1335.
Bird AC . X-linked retinitis pigmentosa. Br J Ophthalmol 1975; 59 (4): 177–199.
Hoare GW . Choroido-retinal dystrophy. Br J Ophthalmol 1965; 49 (9): 449–459.
Falls HF, Cotterman CW . Choroidoretinal degeneration: a sex-linked form in which heterozygous women exhibit a tapetal-like retinal reflex. Arch Ophthalmol 1948; 40: 685–703.
Jacobson SG, Yagasaki K, Feuer WJ, Roman AJ . Interocular asymmetry of visual function in heterozygotes of X-linked retinitis pigmentosa. Exp Eye Res 1989; 48 (5): 679–691.
Berson EL, Rosen JB, Simonoff EA . Electroretinographic testing as an aid in detection of carriers of X-chromosome-linked retinitis pigmentosa. Am J Ophthalmol 1979; 87 (4): 460–468.
Grover S, Fishman GA, Anderson RJ, Lindeman M . A longitudinal study of visual function in carriers of X-linked recessive retinitis pigmentosa. Ophthalmology 2000; 107 (2): 386–396.
Lyon MF . Gene action in the X-chromosome of the mouse (Mus. musculus. L.). Nature 1961; 190: 372–373.
Jay B . X-linked retinal disorders and the Lyon hypothesis. Trans Ophthalmol Soc UK 1985; 104 (Pt 8): 836–844.
Wegscheider E, Preising MN, Lorenz B . Fundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical data. Graefes Arch Clin Exp Ophthalmol 2004; 242 (6): 501–511.
Jacobson SG, Buraczynska M, Milam AH, Chen C, Jarvalainen M, Fujita R et al. Disease expression in X-linked retinitis pigmentosa caused by a putative null mutation in the RPGR gene. Invest Ophthalmol Vis Sci 1997; 38 (10): 1983–1997.
Pomares E, Riera M, Castro-Navarro J, Andres-Gutierrez A, Gonzalez-Duarte R, Marfany G . An intronic single point mutation in RP2 causes semi-dominant X-linked Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 2009.
Khanna H, Hurd TW, Lillo C, Shu X, Parapuram SK, He S et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem 2005; 280 (39): 33580–33587.
Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet 2008; 40 (4): 443–448.
Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI et al. A common allele in RPGRIP1 L is a modifier of retinal degeneration in ciliopathies. Nat Genet 2009, 10 May 2009 [e-pub ahead of print].
Hong DH, Pawlyk BS, Adamian M, Li T . Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci 2004; 45 (1): 36–41.
Zhang Q, Acland GM, Wu WX, Johnson JL, Pearce-Kelling S, Tulloch B et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet 2002; 11 (9): 993–1003.
Dobyns WB, Filauro A, Tomson BN, Chan AS, Ho AW, Ting NT et al. Inheritance of most X-linked traits is not dominant or recessive, just X-linked. Am J Med Genet A 2004; 129 (2): 136–143.
Weleber RG, Butler NS, Murphey WH, Sheffield VC, Stone EM . X-linked retinitis pigmentosa associated with a 2-base pair insertion in codon 99 of the RP3 gene RPGR. Arch Ophthalmol 1997; 115 (11): 1429–1435.
Bauer S, Fujita R, Buraczynska M, Abrahamson M, Ehinger B, Wu W et al. Phenotype of an X-linked retinitis pigmentosa family with a novel splice defect in the RPGR gene. Invest Ophthalmol Vis Sci 1998; 39 (12): 2470–2474.
Andreasson S, Breuer DK, Eksandh L, Ponjavic V, Frennesson C, Hiriyanna S et al. Clinical studies of X-linked retinitis pigmentosa in three Swedish families with newly identified mutations in the RP2 and RPGR-ORF15 genes. Ophthalmic Genet 2003; 24 (4): 215–223.
Andreasson S, Ponjavic V, Abrahamson M, Ehinger B, Wu W, Fujita R et al. Phenotypes in three Swedish families with X-linked retinitis pigmentosa caused by different mutations in the RPGR gene. Am J Ophthalmol 1997; 124 (1): 95–102.
Vervoort R, Wright AF . Mutations of RPGR in X-linked retinitis pigmentosa (RP3). Hum Mutat 2002; 19 (5): 486–500.
Acknowledgements
This study was funded by the Foundation for Fighting Blindness; NIH EY007961. We thank James Friedman, Naheed Khan and Randall Wallach for their helpful discussion in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, D., Khanna, H., Atmaca-Sonmez, P. et al. Long-term follow-up of a family with dominant X-linked retinitis pigmentosa. Eye 24, 764–774 (2010). https://doi.org/10.1038/eye.2009.270
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2009.270
Keywords
This article is cited by
-
X-linked dominant RPGR gene mutation in a familial Coats angiomatosis
BMC Ophthalmology (2021)
-
Novel mutations of RPGR in Chinese families with X-linked retinitis pigmentosa
BMC Ophthalmology (2019)
-
Identification of novel X-linked gain-of-function RPGR-ORF15 mutation in Italian family with retinitis pigmentosa and pathologic myopia
Scientific Reports (2016)