Abstract
Purpose
To identify the prevalence of myocilin gene mutations in a UK glaucoma cohort.
Methods
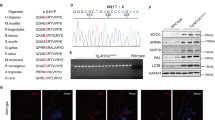
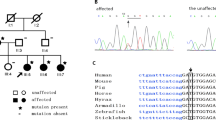
Primary open-angle (POAG) and normal tension glaucoma patients were recruited from the Southampton University Hospital Trust Eye Clinic and satellite regional glaucoma clinics. Phenotype data relating to disease history and other potential risk factors were recorded and blood samples collected for each consenting participant. Point mutation analysis of the myocilin gene was carried out using six overlapping PCR fragments covering the entire coding sequence of the gene. A total of 316 POAG samples were examined of which 7 (2.2 %) tested positive for disease-causing mutations in this gene. One of these seven non-synonymous mutations represented a previously unreported amino-acid substitution of cysteine for arginine at codon 296 (p.R296C) of the myocilin protein.
Conclusions
This study identifies a 2.2% prevalence of myocilin mutations in a cohort of ethnically homogenous glaucoma patients selected from a UK ophthalmic clinic. A novel myocilin mutation is also described. This study identifies that myocilin genetic screening is feasible in NHS glaucoma clinics for genetic counselling and cascade testing of relatives of patients affected by myocilin glaucoma.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Royal College of Ophthalmologists. Royal College of Ophthalmologists. A national research strategy for ophthalmology. RCOphth publication, March 2002.
Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS . Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 2000; 41: 741–748.
Quigley HA . Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393.
Johnson AT, Alward WLM, Sheffield VC, Stone EM . In: Ritch R, Shields MB, Krupin T (eds). The Glaucomas, 2nd ed. St Louis: Mosby, 1996.
Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet 1999; 8: 899–905.
Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL et al. Identification of a gene that causes primary open angle glaucoma. Science 1997; 275: 668–670.
Xie X, Zhou X, Qu X, Wen J, Tian Y, Zheng F . Two novel myocilin mutations in a Chinese family with primary open-angle glaucoma. Mol Vis 2008; 14: 1666–1672.
Wirtz MK, Konstas AG, Samples JR, Kaltsos K, Economou A, Dimopoulos A et al. Myocilin variations and familial glaucoma in Taxiarchis, a small Greek village. Mol Vis 2008; 14: 774–781.
Kanagavalli J, Pandaranayaka E, Krishnadas SR, Krishnaswamy S, Sundaresan P . A review of genetic and structural understanding of the role of myocilin in primary open angle glaucoma. Indian J Ophthalmol 2004; 52: 271–280.
Sripriya S, Uthra S, Sangeetha R, George RJ, Hemamalini A, Paul PG et al. Low frequency of myocilin mutations in Indian primary open-angle glaucoma patients. Clin Genet 2004; 65: 333–337.
Escribano J, Ortego J, Coca-Prados M . Isolation and characterization of cell-specific cDNA clones from a subtractive library of the ocular ciliary body of a single normal human donor: transcription and synthesis of plasma proteins. J Biochem 1995; 118: 921–931.
Fingert JH, Stone EM, Sheffield VC, Alward WL . Myocilin glaucoma. Surv Ophthalmol 2002; 47: 547–561.
Brindle P, Fahey T . Primary prevention of coronary heart disease. Br Med J 2002; 325: 56–57.
Miller SA, Dykes DD, Polesky HF . A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Hewitt AW, Bennett SL, Fingert JH, Cooper RL, Stone EM, Craig JE et al. The optic nerve head in myocilin glaucoma. Invest Ophthalmol Vis Sci 2007; 48: 238–243.
Graul TA, Kwon YH, Zimmerman MB, Kim CS, Sheffield VC, Stone EM et al. A case-control comparison of the clinical characteristics of glaucoma and ocular hypertensive patients with and without the myocilin Gln368Stop mutation. Am J Ophthalmol 2002; 134: 884–890.
Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet 1999; 8: 899–905.
Beeby M, O'Connor BD, Ryttersgaard C, Boutz DR, Perry LJ, Yeates TO . The genomics of disulfide bonding and protein stabilization in thermophiles. PloS Biol 2005; 3: e309.
Acknowledgements
We thank our collaborators Edwin Stone and John Fingert from the University of Iowa for providing positive controls for the MYOC screening. We thank the research nurses of the Wellcome Trust Clinical Research Facility who collected patient information and blood samples and we are very grateful to all patients who kindly agreed to partake in this study. This study was funded by the Gift of Sight Appeal, the International Glaucoma Association and the UK Eire Glaucoma Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ennis, S., Gibson, J., Griffiths, H. et al. Prevalence of myocilin gene mutations in a novel UK cohort of POAG patients. Eye 24, 328–333 (2010). https://doi.org/10.1038/eye.2009.73
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2009.73