Abstract
Purpose
To report two cases of Serratia marcescens endophthalmitis related to presumed aliquot drug contamination, and to determine the incidence of acute endophthalmitis after intravitreal injection of bevacizumab.
Methods
A retrospective chart review of 2020 consecutive intravitreal bevacizumab injection (IVBI) cases at the three affiliated hospitals of Seoul National University (A, B, and C) was carried out between 12 October 2006, and 31 January 2008. Bevacizumab was retrieved multiple times from a single original vial as needed and then discarded on the same day at hospital A and C, or prepared as a single dose aliquot vial at a compounding pharmacy in the hospital B.
Results
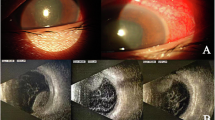
The incidence of endophthalmitis after IVBI was 2/2020 (0.099%). Two patients receiving IVBI on the same day, but by different surgeons in different sites in hospital B, developed acute endophthalmitis. S. marcescens was isolated from the vitreous sample of the two patients. Molecular typing with pulsed field gel electrophoresis showed that the organisms were of the same strain, which suggested that the drug was contaminated at the pharmacy.
Conclusions
Endophthalmitis is a rare complication after IVBI and can be caused by contaminated aliquot drug. Serratia is one of the causative organisms of acute endophthalmitis, which can have devastating consequences, despite the treatment. A compounding pharmacy in a hospital might not be able to guarantee that aliquoted drug is free of contamination for the IVBI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Csaky K . Anti-vascular endothelial factor for neovascular age related macular degeneration. Ophthalmology 2003; 110: 879–881.
Gragoudas ES, Adamis AP, Cunningham Jr ET, Feinsod M, Guyer DR, VISION Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004; 351 (27): 2805–2816.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006; 113 (10): 1695–1705.
Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU . Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 2006; 26 (9): 1006–1013.
Cunningham Jr ET, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005; 112 (10): 1747–1757.
Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006; 26 (9): 999–1005.
Jaissle GB, Ziemssen F, Petermeier K, Szurman P, Ladewig M, Gelisken F et al. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe 2006; 103 (6): 471–475.
Wu L, Arevalo JF, Roca JA, Maia M, Berrocal MH, Rodriguez FJ et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina 2008; 28 (2): 212–219.
Chen HX, Gore-Langton RE, Cheson BD . Clinical trials referral resource: current clinical trials of the anti-VEGF monoclonal antibody bevacizumab. Oncology (Williston Park) 2001; 15 (8): 1017–1026.
Fung AE, Rosenfeld PJ, Reichel E . The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006; 90 (11): 1344–1349.
Jonas JB, Spandau UH, Rensch F, Von Baltz S, Schlichtenbrede F et al. Infectious and noninfectious endophthalmitis after intravitreal bevacizumab. J Ocul Pharmacol Ther 2007; 23 (3): 240–242.
Mason III JO, White MF, Feist RM, Thomley ML, Albert MA, Persaud TO et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina 2008; 28 (4): 564–567.
Meyer CH, Mennel S, Eter N . Incidence of endophthalmitis after intravitreal Avastin injection with and without postoperative topical antibiotic application. Ophthalmologe 2007; 104 (11): 952–957.
Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin(R)): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol 2008; 246 (1): 81–87.
Jonas JB, Spandau Uh, Schlichtenbrede F . Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye 2008; 22: 590–591.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33: 2233–2239.
Pilli S, Kotsolis A, Spaide RF, Slakter J, Freund KB, Sorenson J et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol 2008; 145: 879–882.
Aggio FB, Farah ME, de Melo GB, d'Azevedo PA, Pignatari AC, Höfling-Lima AL . Acute endophthalmitis following intravitreal bevacizumab (Avastin) injection. Eye 2007; 21 (3): 408–409.
Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007; 114: 1860–1867.
Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN . Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991; 98 (5): 639–649.
Kastango ES, American Society of Health-System Pharmacists (ASHP). Blueprint for implementing USP Chapter 797 for compounding sterile preparations. Am J Health Syst Pharm 2005; 62: 1271–1288.
Kumar N, Gandhewar R, Khan MY . Serratia marcescens endophthalmitis after cataract surgery despite vancomycin and gentamicin in irrigation fluid. Can J Ophthalmol 2004; 39 (7): 778–779.
Radda TM . Metastatic Serratia marcescens endophthalmitis. Ophthalmologica 1982; 185 (2): 65–68.
Alkuraya HS, Al-Kharashi AS, Alharthi E, Chaudhry IA . Acute endophthalmitis caused by Staphylococcus lugdunesis after intravitreal bevacizumab (Avastin) injection. Int Ophthalmol 2008; E-pub ahead of print.
Kopel AC, Carvounis PE, Holz ER . Bacillus cereus endophthalmitis following intravitreous bevacizumab injection. Ophthalmic Surg Lasers Imaging 2008; 39 (2): 153–154.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest: None
Rights and permissions
About this article
Cite this article
Lee, S., Woo, S., Park, K. et al. Serratia marcescens endophthalmitis associated with intravitreal injections of bevacizumab. Eye 24, 226–232 (2010). https://doi.org/10.1038/eye.2009.86
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2009.86
Keywords
This article is cited by
-
A Large Outbreak of Fulminant Bacterial Endophthalmitis after Intravitreal Injection of Counterfeit Bevacizumab
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Intravitreal administration of bevacizumab: pros and cons
DARU Journal of Pharmaceutical Sciences (2015)