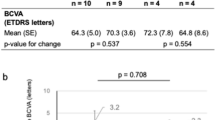
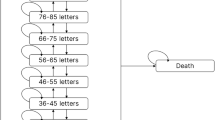
Abstract
The current standard therapy for patients with diabetic macular oedema (DME)—focal/grid laser photocoagulation—usually does not improve impaired vision, and many patients lose vision despite laser therapy. Recent approval of ranibizumab by the European Medicines Agency to treat visual impairment due to DME fulfils the previously unmet medical need for a treatment that can improve visual acuity (VA) in these patients. We reviewed 1- and 2-year clinical trial findings for ranibizumab used as treatment for DME to formulate evidence-based treatment recommendations in the context of this new therapy. DME with or without visual impairment should be considered for treatment when it fulfils the Early Treatment Diabetic Retinopathy Study (ETDRS) criteria for clinically significant oedema. For DME with centre involvement and associated vision loss due to DME, monthly ranibizumab monotherapy with treatment interruption and re-initiation based on VA stability is recommended. Laser therapy based on ETDRS guidelines is recommended for other forms of clinically significant DME without centre involvement or when no vision loss has occurred, despite centre involvement. Because these recommendations are based on randomised controlled trials of 1–2 years duration, guidance may need updating as long-term ranibizumab data become available and as additional therapeutic agents are assessed in clinical trials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aroca PR, Salvat M, Fernandez J, Mendez I . Risk factors for diffuse and focal macular edema. J Diabetes Compl 2004; 18: 211–215.
Ferris III FL, Patz A . Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984; 28 (Suppl): 452–461.
Chen E, Looman M, Laouri M, Gallagher M, Van Nuys K, Lakdawalla D et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin 2010; 26: 1587–1597.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625.
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010; 33: 2399–2405.
Wild S, Roglic G, Green A, Sicree R, King H . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053.
American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern guidelines: Diabetic Retinopathy. American Academy of Ophthalmology: San Francisco, CA, 2010.
The Royal College of Ophthalmologists. Guidelines for diabetic retinopathy. 2005. http://www.rcophth.ac.uk/page.asp?section=451§ionTitle=Cinical+Guidelines, 2011.
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985; 103: 1796–1806.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117: 1064–1077.
Elman MJ, Bressler NM, Qin H, Beck RW, Ferris III FL, Friedman SM et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011; 118: 609–614.
Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S . Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002; 133: 70–77.
Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S . Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003; 110: 1690–1696.
Bhagat N, Grigorian RA, Tutela A, Zarbin MA . Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009; 54: 1–32.
Cunningham Jr ET, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D’Amico DJ et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005; 112: 1747–1757.
Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 2010; 117: 1078–1086.
Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2008; 246: 483–489.
Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008; 115: 1447–1449.
Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009; 116: 1142–1150.
Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ et al. The DA VINCI study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology 2011; 118: 1819–1826.
Novartis Pharma AG. Summary of Product Characteristics 2011. Novartis Pharma AG: Basel, Switzerland, 2011. http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=19409 (accessed 3 March 2011).
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology 2009; 116: 2175–2181.
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV et al. Two-year outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2010; 117: 2146–2151.
Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Boyer DS et al. Aggressive anti-VEGF treatment is necessary to control diabetic macular edema in many patients: month 36 outcomes of the READ2 trial. The Association for Research in Vision and Ophthalmology 2011 Annual Meeting, 1–5 May 2011, p 5332.
Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early treatment diabetic retinopathy study report number 2. early treatment diabetic retinopathy study research group. Ophthalmology 1987; 94: 761–774.
Hansen LL . Visual acuity response to ranibizumab stratified by baseline characteristics in diabetic macular edema (RESOLVE Study). 20th Meeting of the European Association for the Study of Diabetic Eye Complications, 21–22 May 2010.
Kannel WB, McGee DL . Diabetes and cardiovascular risk factors: the Framingham study. Circulation 1979; 59: 8–13.
Cheung N, Mitchell P, Wong TY . Diabetic retinopathy. Lancet 2010; 376: 124–136.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853.
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713.
Singh R, Abhiramamurthy V, Gupta V, Gupta A, Bhansali A . Effect of multifactorial intervention on diabetic macular edema. Diabetes Care 2006; 29: 463–464.
Ryan Jr EH, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S et al. Diabetic macular edema associated with glitazone use. Retina 2006; 26: 562–570.
Liazos E, Broadbent DM, Beare N, Kumar N . Spontaneous resolution of diabetic macular oedema after discontinuation of thiazolidenediones. Diabet Med 2008; 25: 860–862.
Ambrosius WT, Danis RP, Goff Jr DC, Greven CM, Gerstein HC, Cohen RM et al. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye substudy. Arch Ophthalmol 2010; 128: 312–318.
Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, Van Meurs JC, Tanck MW, Oliver N et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 2008; 3: e2675.
Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009; 116: 927–938.
Acknowledgements
We would like to acknowledge Laura Saunderson of Chameleon Communications International, who provided medical writing services with funding from Novartis Pharma AG, Basel, Switzerland. This encompassed preparation of the first draft, referencing, preparing tables and figures, and incorporating authors’ revisions, all under the direction of the authors. At all stages, the authors have had full control over the content of this manuscript, for which they have given final approval and take full responsibility. Novartis Pharma AG enforces a ‘no ghost-writing’ policy. The expert panel meeting was sponsored by Novartis Pharma AG. As funding sponsors, they have had the opportunity to review the manuscript, but do not have authority to change any aspect of the manuscript. Funding for the expert panel meeting and medical writing services was provided by Novartis Pharma AG, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FB is an advisory board member for Alcon, Allergan, Bausch & Lomb, Bayer, Novartis Pharma AG, Pfizer, and Thea. JC-V is a consultant for Novartis, Pfizer, Alcon, Bayer, Allergan, and Astellas Pharma Europe. NVC is a consultant for Novartis Pharma AG, Pfizer, Allergan, Merck, Bayer, and Iridex. GEL received consultancy fees and travel reimbursement from Novartis Pharma AG. PMa received consultancy fees from Novartis Pharma AG, Allergan, Fovea Pharmaceutical, Sanofi Aventis, and Takeda and has also been paid lecture fees by Novartis Pharma AG, Allergan, and Takeda. PMi received consultancy and lecture fees and travel reimbursements from Novartis Pharma AG, Pfizer, Allergan, Solvay (now Abbott), and Bayer. MP received consultancy fees and travel reimbursements from AstraZeneca, Novartis Pharma AG, Pfizer, Roche Diagnostics, and Takeda. CP received consultancy fees and travel reimbursement from Novartis Pharma AG. RS received consultancy fees and travel reimbursement from Novartis Pharma AG. US-E is a consultant for Alcon, Bayer, Novartis, and Pfizer. The University of Vienna performs contract research as a clinical trial centre and Central Reading Centre.
Rights and permissions
About this article
Cite this article
Bandello, F., Cunha-Vaz, J., Chong, N. et al. New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye 26, 485–493 (2012). https://doi.org/10.1038/eye.2011.337
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2011.337
Keywords
This article is cited by
-
Management of Diabetic Macular Edema: Guidelines from the Emirates Society of Ophthalmology
Ophthalmology and Therapy (2022)
-
Review of clinical studies and recommendation for a therapeutic flow chart for diabetic macular edema
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Prognostic role of optical coherence tomography after switch to dexamethasone in diabetic macular edema
Acta Diabetologica (2020)
-
Management of diabetic macular edema in Japan: a review and expert opinion
Japanese Journal of Ophthalmology (2018)
-
The incidence and risk factors for the development of vitreomacular interface abnormality in diabetic macular edema treated with intravitreal injection of anti-VEGF
Eye (2017)