Abstract
Purpose
To determine the efficacy of intravitreal ranibizumab 2.0 mg in patients with recalcitrant neovascular age-related macular degeneration (AMD).
Methods
This single-masked, randomized, prospective, pilot study enrolled patients with subfoveal neovascular AMD. All study eyes had persistent subretinal (SRF) or intraretinal fluid (IRF) on spectral-domain optical coherence tomography (SD-OCT) <30 days following at least 6 monthly intravitreal injections of ranibizumab or bevacizumab. Patients were randomized 2 : 1 to receive either ranibizumab 2.0 or 0.5 mg. Following three-loading treatments 4-weeks apart, both groups were treated using a ‘treat and extend’ regimen guided by eye-tracked SD-OCT through month 12. The primary end point was the mean change in best-corrected visual acuity (BCVA) at month 6.
Results
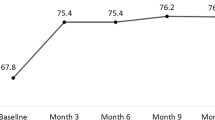
Nine eyes of 9 patients (mean age±SD, 82.0±5.8 years) were enrolled. Seven eyes received ranibizumab 2.0 mg and two eyes received 0.5 mg. Owing to the small number of patients enrolled, no statistical comparison could be made between the two dosages. At month 6, the mean improvement in BCVA was +6.1±3.7 (W=0, P<0.001) ETDRS letters and +2.0 ETDRS letters in the 2.0 and 0.5 mg groups, respectively. In the 2.0 mg group, there was a statistically significant decline in central foveal thickness, SRF and maximum pigment epithelial detachment height at 6 months compared with baseline. No adverse events were reported in either group.
Conclusion
Ranibizumab 2.0 mg has the potential to maintain or improve BCVA in some patients with persistent or recurrent SRF or IRF secondary to neovascular AMD despite prior monthly intravitreal anti-vascular endothelial growth factor therapy with the standard dose.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444.
Freund KB, Zweifel SA, Engelbert M . Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010; 30: 1333–1349.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Group CR, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908.
Engelbert M, Zweifel SA, Freund KB . Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina 2010; 30: 1368–1375.
Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V . Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 2005; 46: 726–733.
Rosenfeld P, Villate N, Feuer Wea . RhuFab V2 (anti-VEGF antibody fragment) in neovascular AMD: safety, tolerability and efficacy of multiple, escalating dose intravitreal injections. Invest Ophthalmol Vis Sci 2003 ARVO 44, E-Abstract 970.
Ho AC . Efficacy and Safety of 2.0 mg and 0.5 mg Ranibizumab in Patients with Subfoveal Age-related Macular Degeneration: The HARBOR Study. American Academy of Ophthalmology: Orlando, USA, 2011.
Chen E, Mariani A, Brown DM . SAVE (Superdose AntiVEgf) Trial - 2.0-mg Intravitreal Ranibizumab for Recalcitrant Neovascular Age-Related Macular Degeneration (Poster). Association for Research in Vision and Ophthalmology: Fort Lauderdale, USA, 2011.
Chan C, Abraham P, Sarraf D, Nuthi ASD, Lin SG, McCannel CA . High-dose (2.0 mg) vs. 0.5 mg Ranibizumab for Treating Vascularized Pigment Epithelial Detachment in Age-Related Macular Degeneration. American Academy of Ophthalmology: Orlando, USA, 2011.
Schaal S, Kaplan HJ, Tezel TH . Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 2008; 115: 2199–2205.
Eghøj MS, Sørensen TL . Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol 2012; 96: 21–23.
Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 2012; 96: 14–20.
Acknowledgements
Peggy Guerrero for assistance co-ordinating the trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This work was supported by the Macula Foundation, Inc. and Genentech, Inc. K Bailey Freund is an advisor to Genentech and Regeneron. Jason S Slakter receives support from Genentech, Regeneron and Novartis; James M Klancnik (Jnr) by Genentech and Regeneron; Richard S Spaide by Genentech.
Rights and permissions
About this article
Cite this article
Fung, A., Kumar, N., Vance, S. et al. Pilot study to evaLuate the role of high-dose rAnibizumab 2.0 mg in the management of neovascular age-related macular degeneration in patients with perSistent/recurrenT macular fluid <30 days following treatment with intravitreal anti-VEGF therapy (the LAST Study). Eye 26, 1181–1187 (2012). https://doi.org/10.1038/eye.2012.174
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2012.174
Keywords
This article is cited by
-
Baseline characteristics and treatment response predictive of nAMD outcomes with ranibizumab therapy in treatment-naive patients: the RACER subgroup analysis
BMC Ophthalmology (2023)
-
Comparison of agents using higher dose anti-VEGF therapy for treatment-resistant neovascular age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab
International Journal of Retina and Vitreous (2021)
-
Outcome of treatment for neovascular age-related macular degeneration by practice-based ophthalmologists compared with a macula clinic
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)
-
Conversion back to bevacizumab or ranibizumab for recurrent neovascular activity with aflibercept in age-related macular degeneration: a case series
International Journal of Retina and Vitreous (2016)