Abstract
Purpose
To analyze the clinical pattern of ocular toxoplasmosis (OT) in a referral centre in Serbia.
Patients and methods
The medical records of consecutive patients admitted for OT to the single referral centre for uveitis in Serbia between 2006 and 2010 were retrospectively analyzed. OT was diagnosed on the basis of typical fundus lesions and positive serology for Toxoplasma.
Results
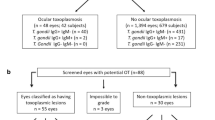
In a total of 457 uveitis patients, OT was the third leading cause, with 59 patients (12.9%). Most OT cases (73%) were monocular. An active primary retinal lesion was observed in 36% and recurrent OT in 64% patients. Localization of lesions was central/paracentral (44%), juxtapapillar (27%), peripheral (19%), and multifocal (10%). Other ocular manifestations of inflammation included vitritis (44%), anterior uveitis (19%), and retinal vasculitis (10%). Complications included choroidal neovascularization in two and exudative retinal detachment with cataract, glaucoma, and cystoid macular oedema in one patient each. The detection of Toxoplasma-specific IgM antibodies in a single patient indicates a low rate of OT concomitant with acute infection. After treatment, the mean best-corrected visual acuity (BCVA) increased significantly. However, 14 (24%) patients ended up legally blind in the affected eye, of which 2 (3%) with bilateral blindness, all with a very poor BCVA (0.047±0.055) at presentation. Visual impairment and treatment outcome were both associated with central localization of lesions (P<0.0001 and P=0.006, respectively).
Conclusion
OT is a significant cause of posterior uveitis in Serbia. Patients should be aware of the recurring nature of OT and react immediately if symptoms occur.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weiss LM, Dubey JP . Toxoplasmosis: A history of clinical observations. Int J Parasitol 2009; 39: 895–901.
Holland GN . Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003; 136: 973–988.
Hogan MJ, Kimura SJ, O’Connor GR . Ocular toxoplasmosis. Arch Ophthalmol 1964; 72: 592–600.
Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier JM, Silveira S et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol 1992; 114: 136–144.
Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A . Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002; 109: 869–878.
Delair E, Monnet D, Grabar S, Dupouy-Camet J, Yera H, Brezin AP . Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am J Ophthalmol 2008; 146: 851–855.
Talabani H, Mergey T, Yera H, Delair E, Brezin AP, Langsley G et al. Factors of occurrence of ocular toxoplasmosis. A review. Parasite 2010; 17: 177–182.
Vallochi AL, Muccioli C, Martins MC, Silveira C, Belfort Jr R, Rizzo LV . The genotype of Toxoplasma gondii strains causing ocular toxoplasmosis in humans in Brazil. Am J Ophthalmol 2005; 139: 350–351.
Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, Belfort Jr R et al. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg Infect Dis 2006; 12: 942–949.
Dubey JP, Velmurugan GV, Chockalingam A, Pena HF, de Oliveira LN, Leifer CA et al. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet Parasitol 2008; 157: 299–305.
Dardé ML . Toxoplasma gondii, ‘new’ genotypes and virulence. Parasite 2008; 15: 366–371.
Bobić B, Nikolić A, Klun I, Vujanić M, Djurković-Djaković O . Undercooked meat consumption remains the major risk factor for Toxoplasma infection in Serbia. Parassitologia 2007; 49: 227–230.
Djurković-Djaković O, Klun I, Khan A, Nikolić A, Knežević-Ušaj S, Bobić B et al. A human origin type II strain of Toxoplasma gondii causing severe encephalitis in mice. Microbes Infect 2006; 8: 2206–2212.
Kitamei H, Kitaichi N, Namba K, Kotake S, Goda C, Kitamura M et al. Clinical features of intraocular inflammation in Hokkaido, Japan. Acta Ophthalmol 2009; 87: 424–428.
de-la-Torre A, Lopez-Castillo CA, Rueda JC, Mantilla RD, Gomez-Marin JE, Anaya JM . Clinical patterns of uveitis in two ophthalmology centres in Bogota, Colombia. Clin Experiment Ophthalmol 2009; 37: 458–466.
Rodriguez A, Calonge M, Pedroza-Seres M, Akova YA, Messmer EM, D’Amico DJ et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol 1996; 114: 593–599.
Khairallah M, Yahia SB, Ladjimi A, Messaoud R, Zaouali S, Attia S et al. Pattern of uveitis in a referral centre in Tunisia, North Africa. Eye (Lond) 2007; 21: 33–39.
Accorinti M, Bruscolini A, Pirraglia MP, Liverani M, Caggiano C . Toxoplasmic retinochoroiditis in an Italian referral center. Eur J Ophthalmol 2009; 19: 824–830.
Jakob E, Reuland MS, Mackensen F, Harsch N, Fleckenstein M, Lorenz HM et al. Uveitis subtypes in a german interdisciplinary uveitis center--analysis of 1916 patients. J Rheumatol 2009; 36: 127–136.
Cimino L, Aldigeri R, Salvarani C, Zotti CA, Boiardi L, Parmeggiani M et al. The causes of uveitis in a referral centre of Northern Italy. Int Ophthalmol 2010; 30: 521–529.
Nguyen AM, Seve P, Le SJ, Gambrelle J, Fleury J, Broussolle C et al. Clinical and etiological aspects of uveitis: a retrospective study of 121 patients referred to a tertiary centre of ophthalmology. Rev Med Interne 2011; 32: 9–16.
de-la-Torre A, Lopez-Castillo CA, Gomez-Marin JE . Incidence and clinical characteristics in a Colombian cohort of ocular toxoplasmosis. Eye (Lond) 2009; 23: 1090–1093.
London NJ, Hovakimyan A, Cubillan LD, Siverio Jr CD, Cunningham Jr ET . Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur J Ophthalmol 2011; 21: 811–819.
Holland GN . Ocular toxoplasmosis: the influence of patient age. Mem Inst Oswaldo Cruz 2009; 104: 351–357.
Garweg JG, Scherrer JN, Halberstadt M . Recurrence characteristics in European patients with ocular toxoplasmosis. Br J Ophthalmol 2008; 92: 1253–1256.
Tugal-Tutkun I, Corum I, Otuk B, Urgancioglu M . Active ocular toxoplasmosis in Turkish patients: a report on 109 cases. Int Ophthalmol 2005; 26: 221–228.
Aleixo AL, Benchimol EI, Neves ES, Silva CS, Coura LC, Amendoeira MR . Frequency of lesions suggestive of ocular toxoplasmosis among a rural population in the State of Rio de Janeiro. Rev Soc Bras Med Trop 2009; 42: 165–169.
Scherrer J, Iliev ME, Halberstadt M, Kodjikian L, Garweg JG . Visual function in human ocular toxoplasmosis. Br J Ophthalmol 2007; 91: 233–236.
Dodds EM, Holland GN, Stanford MR, Yu F, Siu WO, Shah KH et al. Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol 2008; 146: 856–865.
Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N . Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol 2010; 128: 28–32.
Acknowledgements
This work was supported by a project (Grant no. III41019) from the Ministry of Education and Science of Serbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kovačević-Pavićević, D., Radosavljević, A., Ilić, A. et al. Clinical pattern of ocular toxoplasmosis treated in a referral centre in Serbia. Eye 26, 723–728 (2012). https://doi.org/10.1038/eye.2012.20
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2012.20
Keywords
This article is cited by
-
Comparison between the areas of scarred and active toxoplasmic retinochoroiditis
Eye (2021)
-
Chorio-retinal toxoplasmosis: treatment outcomes, lesion evolution and long-term follow-up in a single tertiary center
International Ophthalmology (2020)
-
Assessment of ocular toxoplasmosis patients reported at a tertiary center in the northeast of Iran
International Ophthalmology (2018)