Abstract
Aims
To evaluate the efficacy and safety of intravitreal ranibizumab in patients with choroidal neovascularisation secondary to pathological myopia (myopic CNV). Data are from a pre-planned, 6-month interim analysis.
Methods
Phase II, open-label, single arm, multicentre, 12-month study, recruiting patients (aged ≥18 years) with active primary or recurrent subfoveal or juxtafoveal myopic CNV, with a best-corrected visual acuity (BCVA) score of 24–78 Early Treatment Diabetic Retinopathy Study (ETDRS) letters in the study eye and a diagnosis of high myopia of at least −6 dioptres.
Patients received 0.5 mg ranibizumab administered intravitreally to the study eye, followed by monthly injections given as needed (based on a predefined algorithm) for up to 11 months.
Results
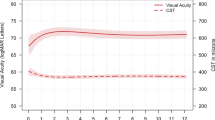
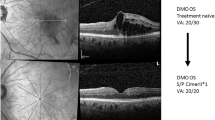
At 6 months, mean BCVA improved from baseline by 12.2 letters, as did central macular thickness (in this interim analysis defined as a measure of either central subfield macular thickness or centre point macular thickness) from baseline by 108 μm in the 48 study eyes of 48 patients. Fewer patients had centre-involving intraretinal oedema (13.0% vs 91.5%), intraretinal cysts (10.9% vs 57.4%), or subretinal fluid (13.0% vs 66.0%) at 6 months than at baseline. Patients received a mean of 1.9 retreatments, were satisfied with ranibizumab treatment, and well being was maintained. No new safety signals were identified.
Conclusions
Results from the planned interim analysis support the role of ranibizumab in the treatment of myopic CNV, with excellent efficacy achieved with a low number of injections and few serious adverse events.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
13 June 2013
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue.
References
Montero JA, Ruiz-Moreno JM . Treatment of choroidal neovascularization in high myopia. Curr Drug Targets 2010; 11 (5): 630–644.
Soubrane G . Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol 2008; 53 (2): 121–138.
Chan WM, Ohji M, Lai TYY, Liu DTL, Tano Y, Lam DSC . Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol 2005; 89 (11): 1522–1528.
Battaglia Parodi M, Iacono P, Bandello F . Antivascular endothelial growth factor for choroidal neovascularization in pathologic myopia. Dev Ophthalmol 2010; 46: 73–83.
Cohen SY . Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina 2009; 29 (8): 1062–1066.
Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 2003; 110 (7): 1297–1305.
Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003; 87: 570–573.
Secretan M, Kuhn D, Soubrane G, Coscas G . Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 1997; 7 (4): 307–316.
Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial-VIP report no. 1. Ophthalmology 2001; 108 (5): 841–852.
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H et alVerteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial--VIP report no. 3.. Ophthalmology 2003; 110 (4): 667–673.
Brown DM, Michels M, Kaiser P, Heier JS, Sy JP, Ianchulev T . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009; 116: 57–65.
Tufail A, Patel P, Egan C, Hykin P, da Cruz L, Gregor Z et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ 2010; 340: c2459.
Ng DS, Kwok AK, Chan CW . Anti-vascular endothelial growth factor for myopic choroidal neovascularization. Clin Experiment Ophthalmol 2012; 40 (1): 98–110.
Calvo-Gonzalez C, Reche-Frutos J, Donate J, Fernandez-Perez C, Garcia-Feijoo J . Intravitreal ranibizumab for myopic choroidal neovascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol 2011; 151: 529–534.
Lucentis Summary of Product Characteristics Electronic Medicines Compendium (Updated 2012; cited 26 June 2012). Available from http://www.medicines.org.uk/EMC/medicine/19409/SPC/Lucentis+10+mg+ml+solution+for+injection/.
Silva RM, Ruiz-Moreno JM, Nascimento J, Carneiro A, Rosa P, Barbosa A et al. Short-term efficacy and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina 2008; 28 (8): 1117–1123.
Konstantinidis L, Mantel I, Pournaras JA, Zografos L, Ambresin A . Intravitreal ranibizumab (Lucentis) for the treatment of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 2009; 247 (3): 311–318.
Mones JM, Amselem L, Serrano A, Garcia M, Hijano M . Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 2009; 23 (6): 1275–1280 quiz 81.
Lai TY, Chan WM, Liu DT, Lam DS . Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina 2009; 29 (6): 750–756.
Mitchell J, Brose LS, Bradley C . Design of a measure of satisfaction with treatment for Macular Degeneration (MacTSQ). Qual Life Res 2007 A-120(Suppl) International Society for Quality of Life Research meeting abstracts, Abstract 1150.
Bradley C, Lewis KS . Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med 1990; 7 (5): 445–451.
Mitchell J, Bradley C . Psychometric evaluation of the 12-item Well-being Questionnaire for use with people with macular disease. Qual Life Res 2001; 10 (5): 465–473.
Acknowledgements
We thank Sue Harris and Sue Cheer representatives of ApotheCom for writing assistance (provision of first manuscript draft), which was funded by Novartis Pharmaceuticals Ltd. (UK). Novartis Pharmaceuticals UK Ltd., Surrey, UK participated in the design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, preparation and review of the manuscript. A Tufail, and PJ Patel have received a proportion of their funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. The views expressed in the publication are those of the authors (AT and PP), and not necessarily those of the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The institutes of the following authors have received funding from Novartis to cover the study-related costs: S Sivaprasad, W Amoaku, S George, A Lotery, A Browning, M McKibbin, and M Majid. W Amoaku has received support for travel to meetings in relation to this study. C Andrews, C Brittain, and A Osborne are employees of Novartis. A Tufail, PJ Patel, M Cole, R Gale, G Menon, and Y Yang have no conflict of interest to disclose.
Additional information
Previous presentation:1. American Academy of Ophthalmology Annual Meeting 2011.2. The Association for Research in Vision and Ophthalmology Annual Meeting 2012.3. The Royal College of Ophthalmology Annual Meeting 2012.
Rights and permissions
About this article
Cite this article
Tufail, A., Patel, P., Sivaprasad, S. et al. Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye 27, 709–715 (2013). https://doi.org/10.1038/eye.2013.8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2013.8
Keywords
This article is cited by
-
Efficacy and safety of different conbercept injection regimens in the treatment of choroidal neovascularization in pathological myopia: a retrospective study
International Ophthalmology (2023)
-
Result of intravitreal aflibercept injection for myopic choroidal neovascularization
BMC Ophthalmology (2021)
-
Pilot study of ziv-aflibercept in myopic choroidal neovascularisation patients
BMC Ophthalmology (2020)
-
Long-term outcomes of the intravitreal injection of ranibizumab for the treatment of choroidal neovascularization secondary to pathologic myopia
International Ophthalmology (2020)
-
OLIMPIC: a 12-month study on the criteria driving retreatment with ranibizumab in patients with visual impairment due to myopic choroidal neovascularization
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)