Abstract
Background
Although anti-VEGF therapy of exudative AMD with bevacizumab and ranibizumab proved efficacious in the majority of patients, CNV activity does not respond to continued treatment after repeated injections in a considerable amount of patients. These are referred to as nonresponders. A change of the drug to bevacizumab or ranibizumab could possibly offer an alternative option for the treatment of nonresponding exudative AMD.
Methods and materials
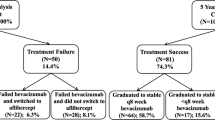
A total of 138 nonresponders who switched therapy from bevacizumab to ranibizumab (n=114) or vice versa (n=24) were included in a retrospective study. Visual acuity (VA) and foveal thickness before and after the switch of therapy were compared. By means of linear regression analysis, we analyzed possible prognostic factors associated with a favorable outcome for visual acuity.
Results
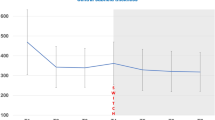
Linear regression analysis revealed a statistically significant benefit for nonresponders when treatment was changed to a different anti-VEGF drug (bevacizumab or ranibizumab). VA at the time of the switch was positively correlated with a beneficial development of VA after changing the drug. There was no significant correlation with age, macular thickness, number of injections before the switch, or the development of VA under treatment before the switch. Both patients switching to Avastin and Lucentis benefitted without statistically significant differences.
Conclusions
An exchange of bevacizumab with ranibizumab or vice versa should be considered in nonresponders in the treatment of exudative AMD. Further prognostic factors may help to identify patients who might benefit from a switch. These factors should be investigated in further studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Velez-Montoya R, Oliver SCN, Olson JL, Fine SL, Mandava N, Quiroz-Mercado H . Current knowledge and trends in age-related macular degeneration: today’s and future treatments. Retina 2012; 33 (8): 1487–1502.
Schmidt-Erfurth U, Pollreisz A, Mitsch C, Bolz M . Antivascular Endothelial Growth Factors in Age-Related Macular Degeneration. In: Bandello F, Battaglia Parodi M, Augustin AJ, Iacono P, Schlingemann RO, Schmidt-Erfurth U, (eds). Developments in Ophthalmology. KARGER: Basel, Switzerland, 2010 pp 21–38.
Klettner A, Roider J . Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci 2008; 49 (10): 4523–4527.
CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364 (20): 1897–1908.
CATT Research Group, Martin DF, Maguire MG, Fine SL, Ying G-S, Jaffe GJ et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; 119 (7): 1388–1398.
IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; 119 (7): 1399–1411.
Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, Weisberger A et al. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology 2012; 119 (5): 1001–1010.
Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, Tokaji E et al. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology 2012; 119 (5): 992–1000.
Dugel PU, Petrarca R, Bennett M, Barak A, Weinberger D, Nau J et al. Macular epiretinal brachytherapy in treated age-related macular degeneration: MERITAGE study: twelve-month safety and efficacy results. Ophthalmology 2012; 119 (7): 1425–1431.
Petrarca R, Dugel PU, Nau J, Slakter JS, Jaffe GJ, Jackson TL . Macular epiretinal brachytherapy in treated age-related macular degeneration (MERITAGE Study): 12 month optical coherence tomography and fluorescein angiography. Ophthalmology 2012; 120 (2): 328–333.
Brown DM, Chen E, Mariani A, Major JC . Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration–primary end point. Ophthalmology 2012; 120 (2): 349–354.
Karagiannis DA, Ladas ID, Parikakis E, Georgalas I, Kotsolis A, Amariotakis G et al. Changing from bevacizumab to ranibizumab in age-related macular degeneration. Is it safe? Clin Interv Aging 2009; 4: 457–461.
Stepien KE, Rosenfeld PJ, Puliafito CA, Feuer W, Shi W, Al-Attar L et al. Comparison of intravitreal bevacizumab followed by ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2009; 29 (8): 1067–1073.
Almony A, Mansouri A, Shah GK, Blinder KJ . Efficacy of intravitreal bevacizumab after unresponsive treatment with intravitreal ranibizumab. Can J Ophthalmol 2011; 46 (2): 182–185.
Kumar N, Marsiglia M, Mrejen S, AT-C Fung, Slakter J, Sorenson J et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 2013; 33 (8): 1605–1612.
Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 2012; 96 (1): 14–20.
Binder S . Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol 2012; 96 (1): 1–2.
Abedi F, Wickremasinghe S, Richardson AJ, Makalic E, Schmidt DF, Sandhu SS et al. Variants in the VEGFA gene and treatment outcome after anti-VEGF treatment for neovascular age-related macular degeneration. Ophthalmology 2013; 120 (1): 115–121.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ehlken, C., Jungmann, S., Böhringer, D. et al. Switch of anti-VEGF agents is an option for nonresponders in the treatment of AMD. Eye 28, 538–545 (2014). https://doi.org/10.1038/eye.2014.64
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2014.64
This article is cited by
-
Granzyme B degrades extracellular matrix and promotes inflammation and choroidal neovascularization
Angiogenesis (2024)
-
JP1, a polypeptide specifically targeting integrin αVβ3, ameliorates choroidal neovascularization and diabetic retinopathy in mice
Acta Pharmacologica Sinica (2023)
-
Real-world experience with brolucizumab in neovascular age-related macular degeneration over 2 years: the REBA extension study
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Cost containment by peer prior authorization program for second line treatment in patients with retinal disease
Israel Journal of Health Policy Research (2021)
-
Stem cell therapies for retinal diseases: from bench to bedside
Journal of Molecular Medicine (2020)