Abstract
Purpose
To compare 2.0 mg ranibizumab (RBZ) injections with 0.5 mg RBZ for eyes with center-involved diabetic macular edema (DME) and a central subfield thickness (CFT) of ≥250 μm on time-domain optical coherence tomography.
Design
Randomized, controlled, multicenter clinical trial.
Methods
Eligible eyes were randomized in a 1:1 ratio to 0.5 mg (n=77) or 2.0 mg (n=75) RBZ. Study eyes received 6-monthly injections.
Main outcome measures
The primary outcome measure was the mean change in best corrected visual acuity (BCVA) at month 6. Secondary outcomes included the incidence and severity of systemic and ocular adverse events and the mean change in CFT from baseline.
Results
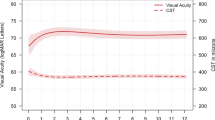
In all, 152 eyes (152 patients) were randomized in the study. At month 6, the mean improvement from baseline BCVA was +9.43 letters in the 0.5 mg RBZ group and +7.01 letters in the 2.0 mg RBZ group (P=0.161). At month 6, one death occurred in the 0.5 mg RBZ group and three deaths in the 2.0 mg RBZ group, all due to myocardial infarction in subjects with a prior history of heart disease. Mean CFT was reduced by 168.58 μm in the 0.5 mg RBZ group and by 159.70 μm in the 2.0 mg RBZ group (P=0.708).
Conclusions
There was no statistically significant difference in the mean number of letters gained between the 0.5 and 2.0 mg RBZ groups through month 6. In this DME study population, high-dose RBZ does not appear to provide additional benefit over 0.5 mg RBZ.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 2006; 142 (6): 961–969.
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009; 116 (11): 2175–81 e1.
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010; 117 (11): 2146–2151.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117 (6): 1064–1077 e35.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118 (4): 615–625.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119 (4): 789–801.
Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol 2013; 131 (2): 139–145.
Acknowledgements
READ-3 was an investigator-sponsored study with the IND (investigative new drug) for the Study held by one of the investigators (QDN). The READ-3 Study was funded by JDRF International with the study drug provided by Genentech, Inc.QDN has received grant support at his institution from Genentech and Regeneron and is a consultant with Santen. DB has received grant support, travel support, and is a consultant with Genentech. He is also a member of the scientific advisory board at Genentech. He is a consultant with Aerpio, Alcon, Allegro, Allergan, Bausch & Lomb, Bayer, GSK, KalVista, Neurotech, Nicox, Novartis, Ohr, Regeneron, Santaris, Santen, and Thrombogenics. He also receives payment for lectures from Alcon and Allergan. He is a stockholder with Allegro, Neurotech, and Ohr. He sits at the scientific advisory board of Alcon, Allergan, Novartis, Neurotech, Pfizer, and Stem Cells, Inc. DC has received grant support at his institution from JDRF. He is a consultant to Alcon, Allergan, and Regeneron; and receives payment for lectures from Allergan. He is a stockholder with ForSight. DM is a consultant with Regeneron and Genentech. LH is a consultant with and has received travel support from Regeneron and Alcon.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
QDN is a recipient of a Physician Scientist Award from Research to Prevent Blindness, New York, NY. PAC is the George S. and Dolores Doré Eccles Professor of Ophthalmology and Neuroscience. QDN is a consultant for Bausch and Lomb and Santen, and has research support from Genentech, MacuSight, Santen, L-Path, Ophthotech, and Regeneron, and has institutional consulting agreement with AbbVie, and honorarium from Bayer, XOMA, Heidelberg, and Quantel. QDN also chairs the Steering Committee for the RISE and RIDE Study, and is on the Steering Committee for the VISTA Study, and other studies sponsored by Genentech and Regeneron. DVD is a consultant for Genentech, Regeneron, Santen, and Allergan and she has received research support from Genentech and Regeneron, and honorarium from Heidelberg and Quantel. DVD also chairs the Steering Committee for the VISTA Study. PAC has institutional consulting agreements with Genentech and GlaxoSmithKline, is a consultant for Allergan, and formerly consulted for Amira, Potentia, and LPath, and served on the data and safety monitoring committee for a phase III trial sponsored by Regeneron, Inc., and has research support from Genentech, Alimera, and Molecular Partners for diabetic macular edema trials and GlaxoSmithKline, Genezyme, and Oxford BioMedica for neovascular AMD trials. These activities are being managed by the Conflict of Interest Committee of the Johns Hopkins University School of Medicine. LH is a stockholder in Covalent Medical LLC and a consultant to Regeneron Pharmaceuticals. DB is a consultant for Allergan, Alcon, Bausch and Lomb, Genentech, Regeneron, Allegro, Neurotech, Ohr, Quantel, Thrombogenics, Allergan, Alcon, Ampio, Thrombogenics, Allegro, Novartis, Roche, and Pfizer. DC is a consultant for Allergan, Alcon, Bausch & Lomb, and Regeneron. DMM is an advisory board member and consultant for Genentech, Regeneron, and Thrombogenics, and receives clinical research support from Genentech, Regeneron and Thrombogenics, Alcon, Allergan, GSK, Pfizer, Acucela, Lpath, Ophthotech, and Quark. The remaining authors declare no conflict of interest.
Additional information
This study was presented in part at the American Academy of Ophthalmology Retina Subspecialty Day, 2012.
Appendix
Appendix
Appendix Investigators, Coordinators, and Staff Members of the READ-3 Study
A. Clinical Sites
1. Wilmer Eye Institute, Johns Hopkins University (Baltimore, MD). PI: Diana V. Do, M.D. Study Coordinator: Jennifer Belz
2. Southeast Retina Center (Augusta, GA). PI: Dennis Marcus, M.D. Study Coordinator: Allison Foster
3. East Bay Retina Institute (Oakland, CA). PI: Eugene Lit, M.D. Study Coordinator: Scotty Renslow
4. Eye Care Specialists (Kingston, PA). PI: Erik Kruger, M.D. Study Coordinator: Patty Yuhas
5. Illinois Retina Associates (Chicago, IL). PI: John Pollack, M.D. Study Coordinator: Barbara Ciscato
6. Retina Group of Florida (Fort Lauderdale, FL). PI: Larry Halperin, M.D. Study Coordinator: Jackie Lopez
7. Retina Institute of Hawaii (Honolulu, HI). PI: Michael Bennett, M.D. Study Coordinator: K'Marie Rego
8. Retina Macula Institute (Torrance, CA). PI: Ron Gallemore, M.D. Study Coordinator: Lillian Chen
9. Retina Vitreous Associates (Beverly Hills, CA). PI: David Boyer, M.D. Study Coordinator: Amanda Tam
10. Texas Retina Associates (Arlington, TX). PI: David Callanan, M.D. Study Coordinator: Sandy Lash
11. Shiley Eye Center, University of California, San Diego (San Diego, CA). PI: Kang Zhang, M.D., Ph.D. Study Coordinator: Maureen Crocker
12. University of Kansas (Kansas City, KS). PI: Andrew Symons, M.D. Study Coordinator: Rebecca Bothwell
13. Black Hills Eye Institute (Rapid City, SD). PI: Prema Abraham, M.D. Study Coordinator: Buffi Green
B. Executive Committee of the READ-3 Study
David Boyer, MD
David G Callanan, MD
Peter A Campochiaro, MD
Quan Dong Nguyen, MD, MSc, Chair
C. Data Safety and Monitoring Committee
Brian P Conway, MD
David J Wilson, MD
D. Reading Center
The Ocular Imaging Research and Reading Center at the Stanley M Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
asir Jamal Sepah, MBBS
Mohammad Ali Sadiq, MD
Mostafa Hanout, MD
E. Data Collection and Monitoring Center
The Ocular Imaging Research and Reading Center at the Stanley M. Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
Salman Sarwar, MD
Jose Maya, MD
Nithya Rajagopalan
Aniruddha Agarwal, MD
F. Coordinating Center
Stanley M Truhlsen Eye Institute, University of Nebraska Medical Center (Omaha, NE)
Mostafa Hanout, MD
Mohamed K Soliman, MD
Lisa Greer, MBA
G. Statistical Analyses
Mohammad Ali Sadiq, MD
Yasir Jamal Sepah, MBBS
Rights and permissions
About this article
Cite this article
Do, D., Sepah, Y., Boyer, D. et al. Month-6 primary outcomes of the READ-3 study (Ranibizumab for Edema of the mAcula in Diabetes—Protocol 3 with high dose). Eye 29, 1538–1544 (2015). https://doi.org/10.1038/eye.2015.142
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2015.142
This article is cited by
-
Differences in the characteristics of subjects achieving complete, partial, or no resolution of macular edema in the READ-3 study
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
A Review of Ranibizumab for the Treatment of Diabetic Retinopathy
Ophthalmology and Therapy (2017)
-
Aflibercept in diabetic macular edema: evaluating efficacy as a primary and secondary therapeutic option
Eye (2016)
-
Diabetic macular oedema: pathophysiology, management challenges and treatment resistance
Diabetologia (2016)
-
Novel Therapies in Development for Diabetic Macular Edema
Current Diabetes Reports (2015)