Abstract
Purpose
To report the visual and anatomic outcomes in eyes with macular oedema (MO) secondary to central retinal vein occlusion (CRVO) that were switched from either intravitreal bevacizumab or ranibizumab to intravitreal aflibercept.
Methods
Two-center retrospective chart review. Eyes with MO secondary to CRVO that received a minimum of three intravitreal injections of bevacizumab or ranibizumab and were switched to intravitreal aflibercept for persistent or recurrent MO not responding to either bevacizumab and/or ranibizumab.
Results
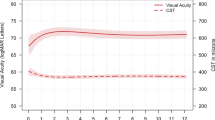
In all 42 eyes of 42 patients were included in the study. The median visual acuity before the switch was 20/126, 1 month after the first injection of aflibercept 20/89 (P=0.0191), and at the end of the follow-up 20/100 (P=0.2724). The median CRT before the switch was 536 μm, 1 month after the first injection of aflibercept 293.5 μm (P=0.0038), and at the end of the follow-up 279 μm (P=0.0013 compared to before the switch). The median number of weeks between injections before the switch was 5.6 and after the switch was 7.6 (P<0.0001).
Conclusion
Converting eyes with refractory MO due to CRVO to aflibercept can result in stabilization of the vision, improved macular anatomy, and extension of the injection interval.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995; 102: 1425–1433.
Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol 2009; 127: 1101.
Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillies M, Heier J et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011; 118: 2453–2460.
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010; 117: 1124–1133.e1.
Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol 2009; 93: 452–456.
Zhang H, Liu Z-L, Sun P, Gu F . Intravitreal bevacizumab for treatment of macular edema secondary to central retinal vein occlusion: eighteen-month results of a prospective trial. J Ocul Pharmacol Ther 2011; 27: 615–621.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011; 118: 2041–2049.
Bhisitkul RB, Campochiaro PA, Shapiro H, Rubio RG . Predictive value in retinal vein occlusions of early vs late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology 2013; 120: 1057–1063.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012; 15: 171–185.
Korobelnik J-F, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO Study. Ophthalmology 2014; 121: 202–208.
Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology 2014; 121: 1414–1420.e1.
Eadie JA, Ip MS, Kulkarni AD . Response to aflibercept as secondary therapy in patients with persistent retinal edema due to central retinal vein occlusion initially treated with bevacizumab or ranibizumab. Retina (Philadelphia, Pa) 2014; 34: 2439–2443.
Boyer D, Heier J, Brown DM, Clark WL, Vitti R, Berliner AJ et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology 2012; 119: 1024–1032.
Holz FG, Roider J, Ogura Y, Korobelnik JF, Simader C, Groetzbach G et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 2013; 97: 278–284.
Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 2013; 155: 429–437.e7.
Ogura Y, Roider J, Korobelnik J-F, Holz FG, Simader C, Schmidt-Erfurth U et al. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the Phase 3 GALILEO Study. Am J Ophthalmol 2014; 158: 1032–1038.e2.
Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T et al. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS ONE 2008; 3: e3554.
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 2013; 156: 29–35.e2.
Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 2013; 156: 15–22.e1.
Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol 2013; 156: 23–28.e2.
Thach AB, Yau L, Hoang C, Tuomi L . Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion: BRAVO and CRUISE trials. Ophthalmology 2014; 121: 1059–1066.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented at the 2015 ARVO meeting in Denver, Colorado, USA and the 2015 ASRS meeting in Vienna, Austria
Rights and permissions
About this article
Cite this article
Papakostas, T., Lim, L., van Zyl, T. et al. Intravitreal aflibercept for macular oedema secondary to central retinal vein occlusion in patients with prior treatment with bevacizumab or ranibizumab. Eye 30, 79–84 (2016). https://doi.org/10.1038/eye.2015.175
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2015.175
This article is cited by
-
Real-World Results of Switching Treatment from Ranibizumab to Aflibercept in Macular Oedema Secondary to Branch Retinal Vein Occlusion
Ophthalmology and Therapy (2018)
-
Leitbild Diagnose und Therapie retinaler Venenverschlüsse
Spektrum der Augenheilkunde (2017)
-
Ranibizumab versus aflibercept for macular edema due to central retinal vein occlusion: 18-month results in real-life data
Graefe's Archive for Clinical and Experimental Ophthalmology (2017)
-
Bevacizumab
Reactions Weekly (2017)
-
Outcomes of switching treatment to aflibercept in patients with macular oedema secondary to central retinal vein occlusion refractory to ranibizumab
International Ophthalmology (2017)