Abstract
Purpose: The aim of this prospective cohort study was to examine uptake and psychological, behavioral, and cognitive outcomes of genetic testing for melanoma risk among individuals with a known family-specific CDKN2A mutation.
Methods: A total of 119 individuals were ascertained via a genetic epidemiological study and completed a series of mailed, self-administered questionnaires at multiple time points, including notification of genetic test availability, and 2 weeks and 12 months after receipt of genetic test results (for “test participants”), or 12 months after notification (for “decliners”).
Results: Since January 2005, 21% of participants (n = 25) have undergone genetic testing, with 75% of those who have received results identified as mutation carriers (n = 15). Factors associated with uptake of genetic counseling included perceived susceptibility to melanoma (odds ratio = 3.60, P = 0.0008), and fatalistic beliefs about melanoma (odds ratio = 0.57, P = 0.005). Compared with baseline, carriers reported significantly reduced anxiety scores at 2 weeks, and reduced depression scores at 2 weeks and 12 months, after receipt of genetic test results. Carriers also reported a significantly greater frequency of clinical skin examination at 12-month follow-up compared with decliners (χ2 = 5.70, P = 0.02). No hypothesis testing was carried out for noncarriers because of their limited number.
Conclusion: These data provide preliminary evidence for healthy psychological, behavioral, and cognitive adjustment after participation in genetic testing for melanoma risk.
Similar content being viewed by others
Main
Germline mutations in two genes, CDKN2A and (rarely) CDK4 have been shown to cause inherited melanoma susceptibility with high penetrance,1–3 and a third such locus has recently been identified on chromosome 1p22.4 In families with multiple cases of the disease, the lifetime risk of developing melanoma for those who carry a pathogenic CDKN2A mutation is estimated to be 58% in Europe, 76% in the United States, and 91% in Australia.2 Gene penetrance estimated by population-ascertained mutation carriers, however, is considerably lower, although still substantial (28% by age 80 years).5 Penetrance seems to be heavily modified by other risk factors such as region of origin2; level of exposure to ultraviolet radiation6,7; melanocortin receptor (MC1R) genotype, a major correlate of skin pigmentation and freckling8,9; and potentially by modifier genes which, in certain families, may also be associated with the presence of atypical nevi.10 Genome-wide association studies have recently begun to identify more common, lower penetrance loci which are associated with melanoma risk at the population level, in addition to the first such example, MC1R.11,12 Further, a subset of CDKN2A mutation carriers also have an increased risk of developing pancreatic cancer (11–17% lifetime risk),13 although there is no evidence for this in Australian families.13 The genetic etiology of melanoma is therefore complex but is coming into sharper focus.
Internationally, there has been much debate about the potential role of genetic testing in families with an inherited pattern of melanoma, as summarized in the consensus statements of the melanoma genetics consortium (GenoMEL),14,15 and by other groups.3,16,17 Although the discovery of mutations in CDKN2A and CDK4 has revolutionized our thinking about familial melanoma, genetic testing for mutations in these genes has yet to penetrate the clinical arena.17 Decisions about genetic testing in this context are likely to be complex given the heterogeneous inheritance pattern, considerable variation in risk caused by the predisposing mutations, and the availability of at least partially effective prevention strategies based on a known major environmental risk factor (i.e., sun exposure). Because of uncertainties about the risk conferred, and the lack of evidence that individuals at high familial risk should be managed differently according to their mutation status, GenoMEL currently recommends that clinical testing for CDKN2A mutations has a very limited role which is confined to highly selected, well-characterized melanoma families.15 Ambivalence about the clinical availability of genetic testing for melanoma risk is also underpinned by a lack of empirical data on the likely consequences of such testing for individuals with an inherited pattern of melanoma.
Genetic testing for melanoma risk has the potential to produce an array of foreseeable benefits and costs. For mutation carriers, the potential benefits of genetic testing include increased information about one's risk status; heightened motivation for preventive health behaviors such as sun protection and skin surveillance; and the possibility of improved survival through earlier melanoma detection.14 But this result also has the potential to cause psychological distress; to engender fatalistic beliefs about melanoma-related outcomes; to encourage overbiopsying of suspicious skin lesions; and to become a basis for discrimination with regard to some types of insurance and/or employment.
In contrast, the likely benefits of genetic testing for individuals found to be noncarriers include reduced melanoma-related anxiety; skin surveillance at a level more appropriately tailored to actual risk status; and for some, freedom from feelings of transmission guilt. Among this group, however, genetic testing may lead to reduced motivation for engagement in preventive behaviors and/or feelings of survivor guilt.18,19 In addition, noncarriers may experience sustained uncertainty about their chances of developing melanoma because the clinical interpretation of a negative test result is unclear given the additional risk associated with phenotype and the potential role of other gene candidates. This situation differs from genetic testing for hereditary breast/ovarian cancer (HBOC) or hereditary nonpolyposis colorectal cancer (HNPCC), where the interpretation of a negative test result in the context of an identified family-specific mutation is well established and, in the case of HNPCC, means relief from intensive screening.20
The existing body of literature on the uptake and outcomes of genetic testing for HBOC and HNPCC may be used to inform the interpretation of findings from similar research in the familial melanoma setting. Specifically, studies involving HBOC and HNPCC show that the most frequently reported reason for undergoing testing is to learn about one's children's cancer risk.21–25 Consistently, several factors have been found to be associated with the decision to undergo genetic testing, including having been affected by cancer,26,27 cancer-related anxiety,28,29 perceived risk,30,31 and strength of family history of disease.26,32 Despite the large volume of published data on uptake of genetic testing for HBOC and HNPCC risk, however, very little is known about the characteristics and beliefs of individuals who choose not to attend a familial cancer clinic (FCC) for genetic counseling. The chief reasons for our limited knowledge in this area are the difficulties inherent in ascertaining individuals who choose not to attend FCCs and/or the lack of interest in participating in research studies among such individuals. In terms of the outcomes associated with testing for HBOC and/or HNPCC, studies demonstrate that noncarriers experience significant psychological benefits from testing (e.g., reduced levels of cancer-specific distress), whereas few adverse effects have been observed among carriers (see Ref. 33 for a summary of this literature).
Previous research conducted by our group has demonstrated a high demand for genetic testing for melanoma risk from those at increased risk.34–36 Whether the potential benefits and risks associated with genetic testing for melanoma risk are realized in practice requires evaluation. A greater understanding of the psychological, behavioral, and cognitive outcomes associated with genetic testing for melanoma risk, for both mutation carriers and noncarriers, is imperative to determining the clinical utility of genetic testing in this context. To our knowledge, this is the first prospective study to examine uptake as well as psychological, behavioral, and cognitive outcomes associated with genetic risk assessment for melanoma. The aims of the present study were 3-fold: (1) to examine uptake of genetic counseling and testing for melanoma risk in a sample of individuals with a strong family history of melanoma and an identified family-specific mutation in the CDKN2A gene; (2) to identify the factors associated with uptake of genetic risk assessment in this context; and (3) to assess the psychological, behavioral, and cognitive outcomes of testing among those who receive a genetic test result compared with those who decline genetic counseling.
It was hypothesized that uptake of genetic risk assessment would be associated with personal history of melanoma,26,37–40 greater number of family members affected by melanoma,26,32 greater perceived importance of the benefits of testing,32,39 higher perceived susceptibility to melanoma,30,31,41 higher melanoma-specific distress at baseline,28–30 and the tendency to seek, or monitor for, risk-related information.42,43 In terms of testing outcomes, we predicted that compared with baseline: (a) carriers would report an increase in psychological distress 2 weeks after receipt of genetic test results, with distress returning to baseline levels at 12-month follow-up33,44 and (b) noncarriers would report decreased levels of psychological distress at 2-week and 12-month follow-up.33,44
METHODS
Participants
Individuals with a strong family history of melanoma (i.e., families comprising at least three relatives with a confirmed melanoma diagnosis) and a known family-specific CDKN2A mutation were ascertained via the Westmead Institute for Cancer Research/University of Sydney center of the Genetic Epidemiology of Melanoma study. This is part of the international GenoMEL consortium (www.genomel.org), a multidisciplinary study of the genetic epidemiology of melanoma.2,45,46 A detailed description of ascertainment into the larger study has been published elsewhere.46 Briefly, multiple-case melanoma families have been ascertained from south eastern Australia to the Sydney arm of this study for over 18 years through (i) a family member who attended the Sydney Melanoma Unit (the largest dedicated melanoma treatment service in the world), the Victorian Melanoma Service, or other clinics, for treatment of melanoma, (ii) referral from health professionals such as clinical geneticists or dermatologists or occasionally, (iii) self-referral after media publicity of melanoma. Data on family structure, cancer history, illness characteristics, skin phenotype, other melanoma risk factors, and genotype are collected as part of this study; however, participants are not systematically provided with any educational materials relating to genetic counseling or testing for melanoma risk. Ineligibility criteria for the present study included having previously undergone genetic testing for melanoma risk, inability to give informed consent, and current diagnosis of metastatic cancer.
Procedure
The appropriate Institutional Review Board gave approval and informed consent was obtained from all participants. Identification of potentially pathogenic mutations in 18 families made genetic testing possible, and in accordance with the larger study protocol, all participating members of these families (N = 176) were informed by letter about the availability of genetic counseling and testing in January 2005 and were simultaneously offered participation in the current study. The initial study invitation package also contained a list of contact details for 15 FCCs located throughout Australia. These FCCs provide a comprehensive service, performing genetic testing in the context of informed consent which includes, but is not limited to, a discussion of the possible test outcomes, their impact on medical management options, their meaning for family members, and their potential socioeconomic and psychological consequences.47 All FCCs within each Australian state use a standardized DNA Consent Form which complies with the clinical practice guidelines of the Human Genetics Society of Australasia.48,49 Pre and posttest genetic counseling is provided by a genetic counselor under the supervision of a medically qualified clinician (i.e., either a geneticist or cancer geneticist), and genetic test results are generally available within 4–6 weeks.
Individuals who did not decline study participation were telephoned 14 days after invitation letters were mailed to determine interest in participating. Up to 10 attempts were made at different times of the day in an effort to contact eligible individuals. Interested individuals were mailed the first (i.e., “baseline”) questionnaire and a prepaid envelope. Participants who chose to undergo genetic testing for melanoma risk (hereafter referred to as “test participants”) received two more questionnaires; one administered 2 weeks and the other 12 months postreceipt of genetic test results. Twelve months after receipt of the initial notification letter, those participants who did not pursue genetic counseling (hereafter referred to as “decliners”) were asked to complete a second questionnaire. Reminder letters and phone calls were made as appropriate to participants who failed to complete the questionnaire within a specified time period. FCC attendance and all genetic test results were verified in three ways: (1) participant self-report; (2) verification of self-report with FCC records; and (3) verification of test results with laboratory reports. Study recruitment ended in December 2005.
Measures
Clinical characteristics were accessed through the local Genetic Epidemiology of Melanoma database and a pedigree was created for each participating family, containing data on: total number of first- and second-degree relatives (FDRs and SDRs) affected by melanoma; total number of FDRs and SDRs deceased because of melanoma; personal history of melanoma; and for participants affected by melanoma, time elapsed since diagnosis and disease stage at clinical presentation.
In the absence of a published algorithm for calculation of lifetime risk of melanoma, estimated risk of being a CDKN2A mutation carrier was calculated for each participant before genetic testing. Given the presence of a family-specific CDKN2A mutation, participants with a personal history of melanoma were assigned an estimated 100% risk of carrying a mutation. Unaffected participants whose closest affected relative was a FDR were assigned a 50% risk, unaffected participants whose closest affected relative was a SDR were assigned a 25% risk, and unaffected participants with no known FDR or SDR with melanoma were assigned an estimated risk of 12.5%.
The baseline questionnaire elicited the following data:
-
1
Demographic characteristics including age, gender, country of origin, marital status, educational level, number of biological children, and occupational environment.
-
2
Perceived susceptibility to melanoma was assessed using five items developed on the basis of previous work.21,34,50 Participants rated their own melanoma risk relative to an average person of the same age and gender, and an average person with a family history of melanoma, using a Likert scale from 1 (“Much lower”) to 5 (“Much higher”). Participants also rated their chances of developing melanoma sometime in the future on a 10 cm visual analogue scale, where 0% represented “no chance” and 100% represented perceived “certainty of developing melanoma,” as well as their expectations (on a 5-point Likert scale) regarding perceived likelihood of developing melanoma in the future, and carrying a genetic mutation. Based on factor analytic results, a summary score was calculated for these items (Cronbach's alpha is 0.73) and used for analysis.
-
3
Endorsement of a genetic model of melanoma was assessed via three items based on our previous work.34,35 Participants rated the importance of each item as a potential cause of melanoma on a 5-point Likert rating scale from 1 (“Not at all important”) to 5 (“Extremely important”). A summary score was calculated for these items and used for analysis.
-
4
Fatalistic beliefs about melanoma: Participants were asked to indicate the degree to which they agreed with the statement, “I believe that melanoma is fatal, even if detected early by regular skin examinations,” using a 5-point Likert scale from 1 (“Strongly disagree”) to 5 (“Strongly agree”). This item was developed on the basis of our previous qualitative work in this area.34,36
-
5
Information-seeking style was assessed using the Miller Behavioral Style Scale.51 Respondents were asked to imagine four hypothetical stress-invoking scenarios of a largely uncontrollable nature. Each scenario was followed by eight responses indicative of either high or low information-seeking (i.e., monitoring) style. According to the Monitoring Process Model, individuals are categorized as high monitors (information-seekers) or low monitors (distractors) on the basis of how they deal with threat-related cues.52 If individuals are forced into their nonpreferred condition, they are likely to experience greater anxiety and reduced compliance to health recommendations, compared with when in their preferred condition.53 Internal consistency of the monitoring scale has been found to be sufficient, with Cronbach's alpha ranging from 0.70 to 0.79.54,55
-
6
Intention to pursue genetic testing for melanoma risk was assessed using a single item with three response options (yes, no, and undecided).
-
7
Perceived benefits and limitations of genetic counseling and testing were assessed using 13 items generated from the published literature,21,22 an expert panel of cancer genetics specialists, and our previous qualitative findings.35,36 Participants were asked to indicate the extent to which each possible benefit or limitation influenced their decision to undergo genetic risk assessment, using a 3-point Likert scale from 0 (“No influence at all”) to 2 (“Strongly influences my decision”). Responses to the seven benefit items were combined to create an overall score for “Perceived Benefits” of testing (range 0–14, internal consistency of 0.79). Scores on the remaining six items were combined into a “Perceived Limitations” subscale (range 0–10, internal consistency of 0.70).
-
8
Melanoma-specific distress: The 15-item Impact of Events Scale (IES) was used to assess melanoma-specific distress.56 Participants rated the frequency of intrusive and avoidant cognitions and behaviors regarding their melanoma risk using a 4-point frequency scale (0, 1, 3, 5) ranging from “Not at all” to “Often.” A score of ≥40 for the total scale is considered indicative of a significant stress response.56,57 Internal consistency for the IES total score was 0.89.
-
9
General anxiety and depression: The Hospital Anxiety and Depression Scale has two 7-item subscales, measuring anxiety and depression.58 Each item has four response options ranging from 0 (“Not at all”) to 3 (“Very much”), yielding scores from 0 to 21 for each subscale. Subscale scores ≥8 indicate potentially elevated distress.58 Internal consistency was 0.85 and 0.74 for the anxiety and depression subscales, respectively.
-
10
Health behaviors: Data on frequency of sunscreen use, skin self-examination (SSE), and clinical skin examination (CSE) were collected via self-report at baseline and 12-month follow-up. During summer, typical use of sunscreen with a sun protection factor of 15+ was recorded on a 5-point Likert scale from 1 (“Never”) to 5 (“Always”).59 SSE was defined as “the careful and deliberate checking for changes in spots or moles on all areas of your skin, including those areas rarely exposed to the sun.”60 Participants indicated their level of engagement in SSE over the past 12 months using a 5-point Likert scale from 1 (“Never”) to 5 (“Once a week”). Participants were also asked to indicate whether they had ever had a CSE and if so, the number of times they had presented for CSE in the past 12 months, giving the month and year of each examination.60
Two weeks after receipt of their genetic test result, test participants completed a second questionnaire comprised of measures 2, 4, 8, and 9. Twelve months after receipt of their result, test participants completed a third questionnaire comprised measures 2, 4, 8–10 and:
-
1
Decision regret: Current experience of regret over the decision to undergo genetic testing was assessed using the 5-item Decision Regret Scale (DRS),61 with response categories ranging from 1 (“Strongly agree”) to 5 (“Strongly disagree”). Scores ranged from 0 to 100, with higher scores indicating greater regret. The DRS has good psychometric properties (Cronbach's alpha of 0.84 in the present study), and correlates strongly with decision satisfaction, decisional conflict, and overall perceived quality of life.61
Decliners completed one follow-up questionnaire 12 months after initial notification of the availability of genetic counseling and testing. This questionnaire contained measures 2, 8–10, and:
-
1
Reasons for not attending genetic counseling: This was assessed using 20 items generated from the published literature,62 an expert panel, and our previous findings.35,36 Participants were asked to indicate the extent to which each possible reason for not attending genetic counseling reflected their experience, using a 4-point Likert scale from 0 (“No agreement”) to 3 (“Completely agree”). Responses to these items were used for descriptive purposes only.
Statistical analysis
Data were analyzed using SPSS 15.0 and SAS 9.1. Differences between participants and nonparticipants for nonpsychological variables were assessed using Pearson χ2 tests, linear-by-linear association tests or t-tests, as appropriate. Pearson χ2 tests were used to examine associations between counseling attendance and categorical predictor variables, whereas t-tests or Mann-Whitney U tests were used, as appropriate, when the predictor variable was continuous. One-way analysis of variance was used when the predictor variable had three or more categories (e.g., estimated CDKN2A mutation carrier risk).
Logistic regression analysis was then used to identify variables that were independently associated with counseling attendance. A forward modeling strategy was employed, starting with the variable with the lowest P value at the bivariate level and adding each variable sequentially, based on its P value, to the model. Models were limited to two predictor variables to avoid the problematic results of logistic models with fewer than 10 events (i.e., participants) per variable.63 Correlations among responses of individuals in the same family cluster were allowed for using generalized estimating equations for logistic regression, thus avoiding family wise errors.64
For carriers and decliners, Wilcoxon tests were used to examine changes in psychological, behavioral, and cognitive outcomes from baseline to each follow-up point. Mann-Whitney U tests were used to compare outcomes of carriers with those of decliners at each time point.
RESULTS
Response rates and analysis of participation bias
Of the 176 individuals considered eligible for study participation, contact details were not available for 10, so 166 individuals were approached for participation. Of these, 119 individuals returned baseline questionnaire data, yielding a response rate of 72% among eligible, successfully contacted participants. Demographic and clinical characteristics of participants are summarized in Table 1. Participants and nonparticipants did not differ significantly by age, gender, personal history of melanoma, or number of relatives affected by melanoma. Over the course of the study, one participant died and 17 participants were lost to follow-up, yielding a retention rate of 85% at study completion. There were no statistically significant differences in age, gender, education level, disease status, perceived risk, or baseline general or melanoma-specific distress between participants who were retained and those lost to follow-up.
Uptake of genetic counseling and testing for melanoma risk
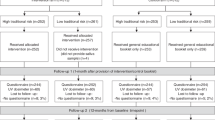
Figure 1 shows the group assessment structure. Up to 3 years after initial notification of the availability of genetic testing for melanoma risk, only 43 of 119 participants (36%) had approached a FCC regarding genetic risk assessment. Of these, 25 participants had blood drawn for the purpose of genetic testing, four participants declined testing after genetic counseling, and 14 participants were not offered testing. Reasons for FCC staff not offering testing included: uncertain clinical utility in the context of the specific mutation and family, or because of the lack of available funding for testing. Thus overall, 21% of participants in this familial melanoma cohort have undergone genetic testing for melanoma risk since initial notification in January 2005. Of the 25 participants who had blood drawn for genetic testing, 15 were identified as carriers (10 previously affected by melanoma, five unaffected), five as noncarriers, and five did not receive their results during the study period because of the lack of available funding for genetic testing for melanoma risk in one Australian state.
Study process and assessment structure. aParticipants made some approach to a familial cancer clinic (either by phone or in-person); however, clinic staff did not offer testing either because of uncertainty about the clinical utility of such testing in the context of the specific mutation and family or because of a lack of available funding for testing. bBecause of the lack of available funding, receipt of test results was delayed. All of these participants opted to have blood drawn for the purpose of genetic testing, and for the familial cancer clinic to store these DNA samples until such funding becomes available.
Testing intentions and expectations at baseline
At baseline, 80 (67%) participants reported an intention to pursue genetic risk assessment, 18 (15%) were undecided, and 20 (17%) had decided not to pursue a genetics consultation. Of the 25 participants who had had blood drawn for genetic testing by the time of study completion (February 2008), 92% had intended to pursue testing at baseline. Of the 94 participants who had not undergone testing, 61% had intended to pursue testing. Perceived carrier status at baseline (i.e., before testing) is presented separately for affected and unaffected participants in Figure 2. Of those with a personal history of melanoma, 86% believed they carried a gene mutation, compared with 39% of unaffected participants.
Perceived benefits and limitations of genetic counseling and testing
Figure 3 shows the rates of endorsement for each item pertaining to the perceived benefits and limitations of genetic risk assessment for melanoma, presented separately for counseling attendees and decliners. Overall, the mean perceived benefits of testing was 7.57 (SD = 3.57), and the mean perceived limitations of testing was 1.67 (SD = 1.91). The benefits most frequently endorsed by the total group (N = 119) as “somewhat” or “very much” influencing participants' decision to pursue testing were: (a) to assist melanoma research (90%); (b) to learn more about my children's risk (82%); and (c) to learn more about the steps I can take to reduce my risk (77%). The most frequently endorsed limitations of testing included (a) concerns about the influence that testing may have on one's family (56%); (b) the belief that testing cannot prevent melanoma onset (26%); and (c) concerns about the difficulties one may have coping with the test result (25%).
Percentage of counseling attendees and decliners endorsing perceived benefits and limitations of genetic risk assessment for melanoma as “somewhat” or “very much” influencing their decision to undergo testing (n = 105). Participants who approached a familial cancer clinic but were not offered genetic risk assessment (n = 14) were excluded from this analysis.
Predictors of actual uptake of genetic counseling
Demographic, clinical, and psychological variables associated with uptake of genetic counseling at the bivariate level are presented in Table 2. Table 3 shows the logistic model predicting uptake of genetic counseling. Overall, the model could discriminate between participants who underwent genetic counseling versus those who declined counseling with 74% accuracy. For every one-unit increase in mean perceived risk, the odds of counseling uptake increased by over 3.5 times (odds ratio [OR] = 3.60, P = 0.0008). A one-unit increase in mean fatalistic beliefs about melanoma, reduced the odds of counseling uptake by approximately 0.5 (OR = 0.57, P = 0.005).
The most common reasons decliners endorsed for not attending genetic counseling are shown in Table 4. Among decliners, those who did not intend to pursue a genetics consultation at baseline (n = 28) more strongly endorsed the following reasons for nonuptake, “I am happy with my life as it is” (P = 0.03), “I don't believe that genetic testing is relevant to me” (P = 0.007), and “Testing cannot tell me when I will develop melanoma” (P = 0.02), compared with participants who had intended to pursue counseling (n = 48).
Decision regret reported by test participants
Twelve months after receipt of genetic test results, the mean score on the DRS was 15.38 (SD = 11.45) for carriers and 6.25 (SD = 7.50) for noncarriers, with overall scores ranging from 0 to 35 of a possible 100. One-quarter of test participants (two carriers, two noncarriers) indicated no decision regret regarding their choice to undergo genetic testing.
Changes in psychological distress over time
At all assessment points, mean psychological distress scores were relatively low for all participant groups, as shown in Table 5. Examining psychological adjustment among carriers, no changes were observed for reported melanoma-specific distress at 2 weeks (P = 0.73) or 12 months (P = 0.26) after receipt of testing results compared with baseline. However, this was not the case for general distress. A reduction in anxiety was observed for carriers 2 weeks after the receipt of testing results (Z = −2.41, P = 0.02). Statistically significant decreases in depression were also found at both short-term (Z = −1.94, P = 0.05) and longer term follow-up points (Z = −2.11, P = 0.04), compared with baseline. Significance tests were not performed for noncarriers because of the limited sample size.
Twelve months after notification of the availability of genetic testing, reductions in general distress were also found among those who declined counseling, with significant decreases observed for both general anxiety (Z = −3.08, P = 0.002) and depression (Z = −2.13, P = 0.03). As reported for carriers, melanoma-specific distress did not change over time among decliners (P = 0.39). Further, at baseline and follow-up, no differences were found between carriers and decliners for melanoma-specific distress, general anxiety or depression (all P values >0.05).
Behavioral and cognitive adjustment over time
Figure 4 illustrates reported sunscreen use, SSE, CSE, and perceived risk at baseline and 12-month follow-up for carriers, noncarriers, and decliners. Sunscreen use, frequency of reported CSE, and perceived risk remained relatively stable over time for all groups. Increases in frequency of reported SSE were observed for all groups at 12-month follow-up, with this increase reaching statistical significance for decliners (Z = −2.03, P = 0.04). Significance tests were not performed for noncarriers because of the limited sample size. Descriptively, however, the findings presented in Figure 4 suggest that although noncarriers seem to have lower subjective estimates of personal susceptibility to melanoma 12-months after receipt of a negative test result, the frequency of behaviors such as sunscreen use, SSE, and CSE may remain relatively stable.
Differences in behavioral and cognitive adjustment between carriers and decliners
Carriers reported significantly greater perceived susceptibility to melanoma compared with decliners both at baseline (Z = −2.65, P = 0.008) and at 12-month follow-up (Z = −2.72, P = 0.007). At baseline, no differences were found between the two groups for reported sunscreen use (P = 0.30), SSE (P = 0.16), or frequency of annual CSE (P = 0.17). At 12-month follow-up, no differences were found between carriers and decliners for reported sunscreen use (P = 0.09) or SSE (P = 0.26). A significant difference was found, however, for annual CSE (χ2 = 5.90, P = 0.02), with 86% of carriers reporting at least one CSE in the 12 months after receipt of genetic test results, compared with only 50% of decliners.
DISCUSSION
This prospective cohort study revealed that, despite relatively high levels of interest in genetic testing for melanoma risk, less than one quarter of participants with an identified, family-specific CDKN2A mutation actually underwent genetic testing over a 3-year period. In comparison with previously reported uptake rates for HBOC and HNPCC, where the percentage of eligible individuals who have opted for testing is approximately 50%,33,45,65 uptake of genetic testing for melanoma risk seems low. These results raise important questions regarding the perceived utility of genetic testing among those with an inherited pattern of melanoma. It is likely that we are beginning to see differences in counseling and test uptake based on the particular condition involved and test candidates' subjective beliefs about its etiology, treatment, and prevention. In contrast to HBOC and HNPCC, for example, melanoma is a cancer where behavioral modification and self-screening are vitally important. For those with a strong family history of melanoma, these actions may be perceived as a priority over genetic risk assessment, particularly given that a negative test result does not provide relief from screening or sun protection practices. Also, in contrast to familial melanoma, an individual's mutation status has major implications for clinical risk management in the context of HBOC and HNPCC. For example, carriers of BRCA1 and/or BRCA2 mutations might consider prophylactic surgery, whereas carriers of HNPCC-related mutations might decide to undergo regular colonoscopy to reduce their cancer risk. Taken together, these factors may limit the perceived utility of genetic testing for melanoma risk among Australian test candidates. In the present study, however, the perceived limitations of testing were found to be low and had no association with actual uptake of genetic risk assessment.
From a clinical perspective, our data also suggest a potential need to address issues of accessibility to cancer genetics services (Table 4). The prospect of having to travel long distances to access a FCC may have deterred those participants who were ambivalent about genetic counseling and testing. In the future, telehealth technology may play an increasingly important role in improving access to genetics services, particularly for those living in rural or regional locations. Access may be particularly important if people are ambivalent about the utility of genetic testing for melanoma risk, in contrast to other familial cancers where psychological barriers have been found to be stronger deterrents to test uptake than accessibility or cost.40
In terms of the perceived benefits of genetic testing for melanoma risk, almost all participants viewed test uptake as a means of driving melanoma research. This substantiates our previous finding that a considerable proportion of individuals with a strong family history of melanoma believe that genetic testing will one day lead to a cure for melanoma, and that this cure will be in the form of “genetic knockout” technology.35 It is important that altruistic motivations such as these are openly discussed within the context of pretest genetic counseling, with a view to clarifying any misconceptions that may arise. Other frequently endorsed motivations for genetic testing included “to learn more about my children's risk” and “to take steps to reduce my risk,” and these findings bear striking similarity to attitudes toward genetic testing for other hereditary cancers.21–25,32
Despite the large volume of published data on uptake of genetic testing for HBOC and HNPCC risk, very little is known about the characteristics and beliefs of individuals who choose not to attend a cancer genetics service for genetic counseling. In the present study, uptake of genetic counseling for melanoma risk was associated with a number of demographic, clinical, and psychological characteristics at the bivariate level, including personal history of melanoma, higher perceived susceptibility to melanoma, greater melanoma-specific distress at baseline, and fewer deaths because of melanoma in the family (Table 2). Although a common finding, it is interesting that unaffected individuals were less likely to undergo genetic counseling compared with affected participants, given the relatively large subset of unaffected individuals (42%) who were uncertain about their carrier status at baseline (Fig. 2). It is possible that attitudes toward uncertainty may moderate intentions to pursue genetic risk assessment, in that individuals with a greater need for certainty may be more likely to undergo counseling and testing compared with those without a need for certainty.66,67 In future research, a focus on attitudes toward uncertainty among individuals offered genetic counseling and CDKN2A mutation testing might provide greater insight into why a higher rate of uptake was not observed in the present sample.
At the multivariate level, when several predictor variables and potentially confounding variables were considered simultaneously, fatalistic beliefs about melanoma were found to reduce the chances of counseling attendance—a novel result in the hereditary cancer context. According to Lazarus and Folkman's Transactional Model of Stress and Coping,68 the ways in which an individual responds to a threat-related situation depends on a number of subjective, cognitive judgments. For example, when an individual learns of the availability of genetic testing for melanoma risk, he or she is likely to assess the impact of such testing on their life (primary appraisal). Based largely on past experiences, the individual will also consider what can be done about the situation (secondary appraisal). Included in these secondary appraisals are the uncertainty that the individual will develop melanoma, perceptions of the severity of melanoma, and the degree to which the individual feels in control of melanoma-related outcomes. Those with a more fatalistic attitude toward melanoma may be less likely to pursue genetic risk assessment because they perceive melanoma-related outcomes as uncontrollable and as such, genetic testing may seem futile. The relationship between perceived control, perceived severity, and uptake of genetic counseling and testing for melanoma risk merits further investigation in future studies. It is also imperative that future research examines the influence of fatalism on sun protection and skin surveillance behaviors in this high-risk population.
The present study is unique in that it also assessed the emotional, behavioral, and cognitive outcomes associated with genetic testing for melanoma risk. Contrary to initial hypotheses, we found short- and longer-term reductions in anxiety and depression among carriers after the receipt of genetic test results. Although we did not observe changes in melanoma-specific distress over time, mean IES scores were remarkably low at all time points, as shown in Table 5. In terms of cognitive and behavioral outcomes among carriers, mean perceived susceptibility to melanoma remained relatively high and stable over time, and no changes were observed in frequency of sunscreen use, SSE, or CSE 12 months after receipt of positive test results. Carriers did report, however, a significantly greater frequency of CSE at 12-month follow-up compared with decliners.
Taken together, these data suggest that Australian CDKN2A mutation carriers experience healthy psychological and behavioral adjustment to the receipt of information about their genetic risk status. In fact, it is possible that carriers may derive emotional benefits from genetic testing for melanoma risk. Positive test results may also serve to justify and/or motivate adherence to biannual doctor consultation for total-body skin examination. Future studies with larger samples are needed to replicate these findings for carriers and clarify the trajectory of behavioral and psychological responses to genetic testing for noncarriers. The clinical utility of genetic testing for noncarriers remains unclear, and more research addressing this issue is critically needed to provide an evidence base for clinical practice. Further, as shown in Figure 4, it appears there is still much scope for improvement in adherence to CSE among both decliners and noncarriers. Currently, the Australian and New Zealand clinical practice guidelines for the management of melanoma recommend that individuals at high risk of melanoma (including those with a strong family history of the disease) be regularly checked by a clinician with 6-month full body examination supported by total body photography and dermoscopy as required.69 Clearly, suboptimal levels of CSE were detected in the present cohort, with 50% of decliners and noncarriers not adhering to annual CSE.
Unexpectedly, we also found significant reductions in both general anxiety and depression among participants who did not attend genetic counseling during the course of the study. At 12-month follow-up, decliners reported increased frequency of SSE, whereas levels of sunscreen use, CSE, and perceived risk remained relatively stable over time. There are several factors that may account for these findings. First, it is possible that ongoing participation in a psychological study focusing on individuals' health beliefs and experiences may engender positive emotional and behavioral changes; although this was not the intention of the study. There is also the possibility that simply notifying individuals of the identification of a family-specific mutation may rouse heightened vigilance with regard to screening behavior or that other, external factors may have contributed to this pattern of results. Why we observed improvements in SSE but not CSE and sun protection, however, is unclear. Because these data are based on self-report, it is not possible to rule out the influence of response bias; however, the validity of self-reported skin cancer screening practices has been shown to be high.70 In any case, these initial data suggest that individuals with an inherited pattern of melanoma demonstrate healthy psychological adjustment, irrespective of their decision regarding genetic counseling and testing for melanoma risk.
Concluding remarks
The strengths and limitations of this study warrant discussion. To our knowledge, this is one of the first prospective cohort studies to provide data on the psychological, behavioral, and cognitive outcomes of genetic testing for melanoma risk, and it is hoped that these findings will facilitate widespread discussion of, and patient education about, the benefits, risks, and limitations associated with such testing. Given the limited available data on the characteristics and beliefs of those individuals who choose not to attend a familial cancer service for genetic counseling, the findings of this study make an important contribution to the literature by shedding light on the factors that may impede or prevent clinic attendance. The high participation and follow-up rates (72% and 85%, respectively), and the lack of differences between participants and nonparticipants, as well as those lost to follow-up and those retained, are also strengths of the study. However, even though we approached all eligible individuals and collected uptake data from all Australian FCCs, the limited number of participants who underwent genetic risk assessment precluded statistical testing among noncarriers, as well as analysis of potential differences in outcomes between carriers and noncarriers. Also, the limited sample size may not have yielded the power to detect potentially clinically important differences between groups and thus, caution should be taken in interpreting the study findings until a larger cohort is followed. Given the rarity of familial melanoma, as data on the implications of genetic testing for this condition accumulates, large-scale international studies and/or meta-analyses will be more powerful strategies for examining psychological and behavioral outcomes. Clinically, identifying the characteristics of those most likely to request genetic assessment for melanoma risk, and the diverse implications of genetic testing for both the individual and his or her family, may enhance the effectiveness of pretest education and counseling, as well as more widespread public health messages about genetic testing for melanoma risk.
References
Hayward N . Genetics of melanoma predisposition. Oncogene 2003; 22: 3053–3062.
Bishop D, Demenais F, Goldstein A, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst 2002; 94: 894–903.
Kefford R, Mann G . Is there a role for genetic testing in patients with melanoma?. Curr Opin Oncol 2003; 15: 157–161.
Gillanders E, Juo S, Holland E, et al. Localization of a novel melanoma susceptibility locus to 1p22. Am J Hum Genet 2003; 73: 301–313.
Begg CB, Orlow I, Hummer AJ, et al. Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst 2005; 97: 1507–1515.
Cannon-Albright L, Meyer L, Goldgar D, et al. Penetrance and expressivity of the chromosome 9p melanoma susceptibility locus (MLM). Cancer Res 1994; 54: 6041–6044.
Goldstein A, Falk R, Fraser M, et al. Sun-related risk factors in melanoma-prone families with CDKN2A mutations. J Natl Cancer Inst 1998; 90: 709–711.
Palmer J, Duffy D, Box N, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype?. Am J Hum Genet 2000; 66: 176–186.
van der Velden P, Sandkuijl L, Bergman W, et al. Melanocortin-1 receptor variant R151C modifies melanoma risk in Dutch families with melanoma. Am J Hum Genet 2001; 69: 774–779.
Tucker M, Fraser M, Goldstein A, Elder DE, Guerry D 4th, Organic SM . The risk of melanoma and other cancers in melanoma-prone families. J Invest Dermatol 1993; 100: 350S–355S.
Brown K, Macgregor S, Montgomery G, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat Genet 2008; 40: 838–840.
Gudbjartsson D, Sulem P, Stacey S, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet 2008; 40: 886–891.
Vasen H, Gruis N, Frants R, van Der Velden PA, Hille ET, Bergman W . Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer 2000; 87: 809–811.
Kefford R, Newton-Bishop J, Bergman W, Tucker M . Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a Consensus Statement of the Melanoma Genetics Consortium. J Clin Oncol 17: 3245–3251. 1999.
Kefford R, Newton-Bishop J, Tucker M, et al. Genetic testing for melanoma. Lancet Oncol 2002; 3: 653–654.
de Snoo F, Bergman W, Gruis N . Familial melanoma: a complex disorder leading to controversy on DNA testing. Familial Cancer 2003; 2: 109–116.
Tsao H, Niendorf K . Genetic testing in hereditary melanoma. J Am Acad Dermatol 2004; 51: 803–808.
Tibben A, Vegter-van der Vlis M, Skraastad MI, et al. DNA-testing for Huntington's disease in The Netherlands: a retrospective study on psychosocial effects. Am J Med Genet 1992; 44: 94–99.
Tibben A, Vegter-vd Vlis M, vd Niermeijer MF, et al. Testing for Huntington's disease with support for all parties. Lancet 1990; 335: 553.
Stanley AJ, Gaff CL, Aittomaki AK, Fabre LC, Macrae FA, St John J . Value of predictive genetic testing in management of hereditary non-polyposis colorectal cancer (HNPCC). Med J Aust 2000; 172: 313–316.
Lerman C, Daly M, Masny A, Balshem A . Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol 1994; 12: 843–850.
Lerman C, Seay J, Balshem A, Audrain J . Interest in genetic testing among first-degree relatives of breast cancer patients. Am J Med Genet 1995; 57: 385–392.
Struewing JP, Lerman C, Kase RG, Giambarresi TR, Tucker MA . Anticipated uptake and impact of genetic testing in hereditary breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev 1995; 4: 169–173.
Lynch HT, Lemon SJ, Durham C, et al. A descriptive study of BRCA1 testing and reactions to disclosure of testing results. Cancer 1997; 79: 2219–2228.
Ho S, Ho J, Chan C, Kwan K, Tsui Y . Decisional consideration of hereditary colon cancer genetic test results among Hong Kong Chinese adults. Cancer Epidemiol Biomarkers Prev 2003; 12: 426–432.
Hadley DW, Jenkins J, Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med 2003; 163: 573–582.
Julian-Reynier J, Sobol H, Sevilla C, Noguès C, Bourret P ; French Cancer Genetic Network. Uptake of hereditary breast/ovarian cancer genetic testing in a French national sample of BRCA1 families. Psycho-Oncol 2000; 9: 504–510.
Lerman C, Schwartz M, Lin TH, Hughes C, Narod S, Lynch HT . The influence of psychological distress on use of genetic testing for cancer risk. J Consult Clin Psychol 1997; 65: 414–420.
Foster C, Evans DG, Eeles R, et al. Non-uptake of predictive genetic testing for BRCA1/2 among relatives of known carriers: attributes, cancer worry, and barriers to testing in a multicenter clinical cohort. Genet Test 2004; 8: 23–29.
Codori AM, Petersen G, Miglioretti D, et al. Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiol Biomarkers Prev 1999; 8: 345–351.
Andrews L, Meiser B, Apicella C, Tucker K . Psychological impact of genetic testing for breast cancer susceptibility in women of Ashkenazi Jewish background: a prospective study. Genet Test 2004; 8: 240–247.
Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer: a prospective study of patient decision making and outcomes. JAMA 1996; 275: 1885–1892.
Meiser B . Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psycho-Oncol 2005; 14: 1060–1074.
Kasparian N, Butow P, Meiser B, Mann G . High- and average-risk individuals' beliefs about, and perceptions of, malignant melanoma: an Australian perspective. Psycho-Oncol 2008; 17: 270–279.
Kasparian N, Meiser B, Butow P, Job R, Mann G . Anticipated uptake of genetic testing for familial melanoma in an Australian sample: an exploratory study. Psycho-Oncol 2007; 16: 69–78.
Kasparian N, Meiser B, Butow P, Job R, Mann GJ . Better the devil you know? High-risk individuals' anticipated psychological responses to genetic testing for melanoma susceptibility. J Genet Couns 2006; 15: 433–447.
Julian-Reynier C, Sobol H, Sevilla C, et al. Uptake of hereditary breast/ovarian cancer genetic testing in a French national sample of BRCA1 families. Psycho-Oncol 2000; 9: 504–510.
Lee SC, Bernhardt B, Helzlsouer K . Utilization of BRCA1/2 genetic testing in the clinical setting: report from a single institution. Cancer 2002; 94: 1876–1885.
Armstrong K, Calzone K, Stopfer J, Fitzgerald G, Coyne J, Weber B . Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev 2000; 9: 1251–1254.
Biesecker BB, Ishibe N, Hadley DW, et al. Psychosocial factors predicting BRCA1/BRCA2 testing decisions in members of hereditary breast and ovarian cancer families. Am J Med Genet 2000; 93: 257–263.
Schwartz M, Hughes C, Roth J, et al. Spiritual faith and genetic testing decisions among high-risk breast cancer probands. Cancer Epidemiol Biomarkers Prev 2000; 9: 381–385.
Miller S, Shoda Y, Hurley K . Applying cognitive-social theory to health protective behavior: breast self-examination in cancer screening. Psychol Bull 1996; 119: 70–94.
Miller S, Fang C, Diefenbach M, Bales C . Tailoring psychosocial interventions to the individual's health information-processing style: the influence of monitoring versus blunting in cancer risk and disease. In: Baum A, Andersen B, editors, Psychosocial interventions for cancer. Washington, DC: American Psychological Society, 2001: 343–362.
Pasacreta J . Psychosocial issues associated with genetic testing for breast and ovarian cancer risk: an integrative review. Cancer Invest 2003; 21: 588–623.
Goldstein A, Chan M, Harland M, et al. Assessment of high-risk melanoma susceptibility genes and their associations with pancreatic cancer, neural system tumors, and uveal melanoma: a Melanoma Genetics Consortium (GenoMEL) study. Cancer Res 2006; 66: 9818–9828.
Holland E, Schmid H, Kefford R, Mann G . CDKN2A (p16INK4a) and CDK4 mutation analysis in 131 Australian melanoma probands: effect of family history and multiple primary melanomas. Genes Chromosomes Cancer 1999; 25: 339–348.
Australian Cancer Network Familial aspects of cancer: a guide to clinical practice. Canberra: National Health and Medical Research Council (NH&MRC) of Australia, 1999.
Human Genetics Society of Australasia. HGSA policy document: presymptomatic and predictive testing for genetic disorders. Available at: http://www.hgsa.com.au/images/UserFiles/Attachments/PresymptomaticandPredictiveTestingforGeneticDisordersV22005.pdf. Accessed April 2005.
Human Genetics Society of Australasia. HGSA policy document: process of genetic counseling. Available at: http://www.hgsa.com.au/images/UserFiles/Attachments/PROCESSOFGENETICCOUNSELLINGFINAL.pdf. Accessed August 2008.
Meiser B, Butow P, Barratt A, et al. Breast cancer screening uptake in women at increased risk of developing hereditary breast cancer. Breast Cancer Res Treat 2000; 59: 101–111.
Miller S . Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J Pers Soc Psychol 1987; 52: 345–353.
Miller SM . Monitoring and blunting of threatening information: cognitive interference and facilitation in the coping process. In: Sarason I, Pierce G, Sarason B, editors, Cognitive interference: theories, methods, and findings. Hillsdale, NJ: Lawrence Erlbaum Associates, 1996; 175–190.
Miller S, Roussi P, Buzaglo J, et al. Enhanced counselling for women undergoing BRCA1/2 testing: impact on subsequent decision making about risk reduction behaviours. Health Educ Behav 2005; 32: 654–667.
Miller S, Roussi P, Altman D, Helm W, Steinberg A . Effects of coping style on psychological reactions of low-income, minority women to colposcopy. J Reprod Med 1994; 39: 711–718.
Muris P, Schouten E . Monitoring and blunting: a factor analysis of the Miller Behavioural Style Scale. Pers Ind Diff 1994; 17: 285–287.
Horowitz M, Wilner N, Alvarez W . The Impact of Event Scale: a measure of subjective stress. Psychosom Med 1979; 41: 209–218.
Cella D, Mahon SM, Donovan M . Cancer recurrence as a traumatic event. Behav Med 1990; 16: 15–22.
Zigmond A, Snaith R . The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370.
Turrisi R, Hillhouse J, Gebert C, Grimes J . Examination of cognitive variables relevant to sunscreen use. J Behav Med 1999; 22: 493–509.
Manne S, Fasanella N, Connors J, Floyd B, Wang H, Lessin S . Sun protection and skin surveillance practices among relatives of patients with malignant melanoma: prevalence and predictors. Prev Med 2004; 39: 36–47.
Brehaut J, O'Connor A, Wood T, et al. Validation of a Decision Regret Scale. Med Decis Making 2003; 23: 281–292.
Riedijk S, de Snoo F, van Dijk S, et al. Hereditary melanoma and predictive genetic testing: why not?. Psycho-Oncol 2005; 14: 738–745.
Peduzzi P, Concato J, Kemper E, Holford T, Feinstein A . A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379.
Liang K, Zeger S . Longitudinal data analysis using generalised linear models. Biometrika 1986; 73: 13–22.
Ropka M, Wenzel J, Phillips E, Siadaty M, Philbrick J . Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev 2006; 15: 840–855.
Braithwaite D, Sutton S, Steggles N . Intention to participate in predictive genetic testing for hereditary cancer: the role of attitude toward uncertainty. Psychol Health 2002; 17: 761–772.
Croyle R, Dutson D, Tran V, Yi-Chun S . Need for certainty and interest in genetic testing. Women's Health 1995; 1: 329–339.
Folkman S . Personal control and stress and coping processes: a theoretical analysis. J Pers Soc Psychol 1984; 46: 839–852.
Australian Cancer Network Clinical practice guidelines for the management of melanoma in Australia and New Zealand. Canberra: National Health and Medical Research Council (NHMRC) 2008.
Aitken J, Youl P, Janda M, et al. Validity of self-reported skin screening histories. Am J Epidemiol 2004; 159: 1098–1105.
Acknowledgements
N.A.K. is supported by a Clinical Post Doctoral Research Fellowship from the National Health and Medical Research Council of Australia (NH&MRC, ID 510399). B.M. is supported by a Career Development Award from the NH&MRC (ID 350989). P.N.B. is supported by a Research Fellowship from the NH&MRC (ID 211199). Genetic epidemiology studies of the cohort have been supported by Grants from the NH&MRC (ID 211172, 402761), the Cancer Councils of New South Wales, Queensland and Victoria, and the US National Institutes of Health (RO1 83115-01A2). This project was also supported by The Cancer Council NSW Strategic Research Partnership Grant (ID SRP06-X5), a Cancer Institute NSW Program Grant for Excellence in Translational Research, and a European Commission Framework 6 Program Grant (ID 018702) to GenoMEL. The authors acknowledge the valuable contribution of all of the individuals who participated in this research. They thank Jenny Leary for providing confirmation of all genetic test results reported in this study, as well as Kathy Tucker, Helen Schmid, Sophie Devery, Nikki Gefland, Judy Kirk, Poonam Zodgekar, Rachel Williams, Carolyn James, Michael Gattas, Janet Tyler, Belinda Creighton, Margaret Gleeson, Rebecca D'Souza, Graeme Suthers, and Mac Gardner for assistance with data collection associated with clinic attendance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kasparian, N., Meiser, B., Butow, P. et al. Genetic testing for melanoma risk: a prospective cohort study of uptake and outcomes among Australian families. Genet Med 11, 265–278 (2009). https://doi.org/10.1097/GIM.0b013e3181993175
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1097/GIM.0b013e3181993175
Keywords
This article is cited by
-
FRAMe: Familial Risk Assessment of Melanoma—a risk prediction tool to guide CDKN2A germline mutation testing in Australian familial melanoma
Familial Cancer (2021)
-
A cluster randomized controlled trial of an online psychoeducational intervention for people with a family history of depression
BMC Psychiatry (2019)
-
The PiGeOn project: protocol for a longitudinal study examining psychosocial, behavioural and ethical issues and outcomes in cancer tumour genomic profiling
BMC Cancer (2018)
-
The PiGeOn project: protocol of a longitudinal study examining psychosocial and ethical issues and outcomes in germline genomic sequencing for cancer
BMC Cancer (2018)
-
Development of an Educational Program Integrating Concepts of Genetic Risk and Preventive Strategies for Children with a Family History of Melanoma
Journal of Cancer Education (2018)