Abstract
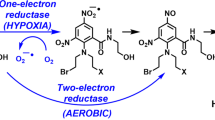
Two of the successful gene-directed enzyme prodrug therapies include herpes simplex virus–thymidine kinase (HSV1–TK) enzyme-ganciclovir prodrug and the Escherichia coli nitroreductase (NTR) enzyme-CB1954 prodrug strategies; these enzyme-prodrug combinations produce activated cytotoxic metabolites of the prodrugs capable of tumor cell death by inhibiting DNA synthesis and killing quiescent cells, respectively. Both these strategies also affect significant bystander cell killing of neighboring tumor cells that do not express these enzymes. We have developed a dual-combination gene strategy, where we identified HSV1-TK and NTR fused in a particular orientation can effectively kill tumor cells when the tumor cells are treated with a fusion HSV1-TK–NTR gene– along with a prodrug combination of GCV and CB1954. In order to determine whether the dual-system demonstrate superior therapeutic efficacy than either HSV1-TK or NTR systems alone, we conducted both in vitro and in vivo tumor xenograft studies using triple negative SUM159 breast cancer cells, by evaluating the efficacy of cell death by apoptosis and necrosis upon treatment with the dual HSV1-TK genes-GCV-CB1954 prodrugs system, and compared the efficiency to HSV1-TK–GCV and NTR-CB1954. Our cell-based studies, tumor regression studies in xenograft mice, histological analyses of treated tumors and bystander studies indicate that the dual HSV1-TK–NTR–prodrug system is two times more efficient even with half the doses of both prodrugs than the respective single gene-prodrug system, as evidenced by enhanced apoptosis and necrosis of tumor cells in vitro in culture and xenograft of tumor tissues in animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Both GW . Recent progress in gene-directed enzyme prodrug therapy: an emerging cancer treatment. Curr Opin Mol Ther 2009; 11: 421–432.
Niculescu-Duvaz I, Springer CJ . Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol 2005; 30: 71–88.
Springer CJ, Niculescu-Duvaz I . Prodrug-activating systems in suicide gene therapy. J Clin Invest 2000; 105: 1161–1167.
Bhaumik S, Sekar TV, Depuy J, Klimash J, Paulmurugan R . Noninvasive optical imaging of nitroreductase gene-directed enzyme prodrug therapy system in living animals. Gene Therapy 2012; 19: 295–302.
Knox RJ, Friedlos F, Jarman M, Roberts JJ . A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem Pharmacol 1988; 37: 4661–4669.
Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK . Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer 1995; 31A: 2362–2370.
Bhaumik S . Advances in imaging gene-directed enzyme prodrug therapy. Curr Pharm Biotechnol 2011; 12: 497–507.
Morris JC, Ramsey WJ, Wildner O, Muslow HA, Aguilar-Cordova E, Blaese RM . A phase I study of intralesional administration of an adenovirus vector expressing the HSV-1 thymidine kinase gene (AdV.RSV-TK) in combination with escalating doses of ganciclovir in patients with cutaneous metastatic malignant melanoma. Hum Gene Ther 2000; 11: 487–503.
Mailly L, Leboeuf C, Tiberghien P, Baumert T, Robinet E . Genetically engineered T-cells expressing a ganciclovir-sensitive HSV-tk suicide gene for the prevention of GvHD. Curr Opin Investig Drugs 2010; 11: 559–570.
Crystal RG, Hirschowitz E, Lieberman M, Daly J, Kazam E, Henschke C et al. Phase I study of direct administration of a replication deficient adenovirus vector containing the E. coli cytosine deaminase gene to metastatic colon carcinoma of the liver in association with the oral administration of the pro-drug 5-fluorocytosine. Hum Gene Ther 1997; 8: 985–1001.
Cunningham C, Nemunaitis J . A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version. Hum Gene Ther 2001; 12: 1594–1596.
Corban-Wilhelm H, Ehemann V, Becker G, Greulich D, Braun K, Debus J . Comparison of different methods to assess the cytotoxic effects of cytosine deaminase and thymidine kinase gene therapy. Cancer Gene Ther 2004; 11: 208–214.
McNeish IA, Green NK, Gilligan MG, Ford MJ, Mautner V, Young LS et al. Virus directed enzyme prodrug therapy for ovarian and pancreatic cancer using retrovirally delivered E. coli nitroreductase and CB1954. Gene Therapy 1998; 5: 1061–1069.
Bridgewater JA, Knox RJ, Pitts JD, Collins MK, Springer CJ . The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite. Hum Gene Ther 1997; 8: 709–717.
Dachs GU, Tupper J, Tozer GM . From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anticancer Drugs 2005; 16: 349–359.
Palmer DH, Mautner V, Mirza D, Oliff S, Gerritsen W, van der Sijp JR et al. Virus-directed enzyme prodrug therapy: intratumoral administration of a replication-deficient adenovirus encoding nitroreductase to patients with resectable liver cancer. J Clin Oncol 2004; 22: 1546–1552.
Patel P, Young JG, Mautner V, Ashdown D, Bonney S, Pineda RG et al. A phase I/II clinical trial in localized prostate cancer of an adenovirus expressing nitroreductase with CB1954 [correction of CB1984]. Mol Ther 2009; 17: 1292–1299.
Searle PF, Chen MJ, Hu L, Race PR, Lovering AL, Grove JI et al. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin Exp Pharmacol Physiol 2004; 31: 811–816.
McBride WH . Integration of adenovirus thymidine kinase suicide-gene therapy with surgery and radiation therapy for malignant glioma. Future Oncol 2012; 8: 17–20.
Schwarzenberger P, Byrne P, Gaumer R, Norton J, Harrison L, Marrogi A et al. Treatment of mesothelioma with gene-modified PA1STK cells and ganciclovir: a phase I study. Cancer Gene Ther 2011; 18: 906–912.
Brade AM, Szmitko P, Ngo D, Liu FF, Klamut HJ . Heat-directed suicide gene therapy for breast cancer. Cancer Gene Ther 2003; 10: 294–301.
Corban-Wilhelm H, Becker G, Bauder-Wust U, Greulich D, Debus J . Cytosine deaminase versus thymidine kinase: a comparison of the antitumor activity. Clin Exp Med 2003; 3: 150–156.
Jia W, Mei L, Wang Y, Liu L, Che G . Double suicide genes selectively kill human umbilical vein endothelial cells. Virol J 2011; 8: 74.
Luo XR, Li JS, Niu Y, Miao L . Targeted killing effects of double CD and TK suicide genes controlled by survivin promoter on gastric cancer cell. Mol Biol Rep 2011; 38: 1201–1207.
van der Eb MM, Geutskens SB, van Kuilenburg AB, van Lenthe H, van Dierendonck JH, Kuppen PJ et al. Ganciclovir nucleotides accumulate in mitochondria of rat liver cells expressing the herpes simplex virus thymidine kinase gene. J Gene Med 2003; 5: 1018–1027.
Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res 2003; 63: 7497–7506.
Uckert W, Kammertons T, Haack K, Qin Z, Gebert J, Schendel DJ et al. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther 1998; 9: 855–865.
Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL et al. The ‘bystander effect’: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 1993; 53: 5274–5283.
Djeha AH, Hulme A, Dexter MT, Mountain A, Young LS, Searle PF et al. Expression of Escherichia coli B nitroreductase in established human tumor xenografts in mice results in potent antitumoral and bystander effects upon systemic administration of the prodrug CB1954. Cancer Gene Ther 2000; 7: 721–731.
Amano S, Gu C, Koizumi S, Tokuyama T, Namba H . Tumoricidal bystander effect in the suicide gene therapy using mesenchymal stem cells does not injure normal brain tissues. Cancer Lett 2011; 306: 99–105.
Acknowledgements
We thank Dr Srabani Bhaumik from GE Global Research for her support, and providing CytoCy5S substrate for imaging NTR enzyme. We also thank Dr Tim Doyle for support in providing training for the use of different Imaging instruments from Stanford Small Animal Imaging Facility. We thank Dr Sanjiv Sam Gambhir, Chairman, Department of Radiology, Stanford University for providing helpful support and facility for conducting this research. We thank Dr Chaitali Banerjee for her input in preparing this paper. We thank the department of Radiology, Stanford University for funding support (Ramasamy Paulmurugan), and the Canary center at Stanford for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Sekar, T., Foygel, K., Willmann, J. et al. Dual-therapeutic reporter genes fusion for enhanced cancer gene therapy and imaging. Gene Ther 20, 529–537 (2013). https://doi.org/10.1038/gt.2012.66
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/gt.2012.66
Keywords
This article is cited by
-
The effect of gene therapy on postoperative recurrence of small hepatocellular carcinoma (less than 5cm)
Cancer Gene Therapy (2019)