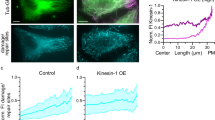
Abstract
Microtubule-based transport is required for plasmid translocation to the nucleus during transfections, and having stable structures could enhance this movement. In previous studies, in which the cytoskeleton was disrupted, we found that populations of microtubules remain that are stable and highly acetylated. By increasing the levels of acetylated tubulin through inhibition of the tubulin deacetylase HDAC6, we observe more rapid plasmid nuclear localization of transfected plasmids and greater levels of gene transfer. In this study, we sought to understand plasmid movement in cells with enhanced tubulin acetylation. Using variations of a microtubule spin-down assay, we found that plasmids bound to hyper-acetylated microtubules to a greater degree than they did to unmodified microtubules. To determine whether microtubule acetylation also affects cytoplasmic trafficking, plasmid movement was evaluated in real time by particle tracking in cells with varying levels of acetylated microtubules. We found that plasmids display greater net rates of movement, spend more time in productive motion and display longer runs of continuous motion in cells with highly acetylated microtubules compared with those with fewer modifications. These results all suggest that plasmid movement is enhanced along highly acetylated microtubules, reducing the time spent in the cytoplasm before nuclear import. Taken together, these findings provide a foundation for determining how modulation of microtubule acetylation can be used as a means to increase intracellular trafficking of plasmids and enhance gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lechardeur D, Lukacs GL . Intracellular barriers to non-viral gene transfer. Curr Gene Ther 2002; 2: 183–194.
Zhou R, Geiger RC, Dean DA . Intracellular trafficking of nucleic acids. Expert Opin Drug Deliv 2004; 1: 127–140.
Lechardeur D, Lukacs GL . Nucleocytoplasmic transport of plasmid DNA: a perilous journey from the cytoplasm to the nucleus. Hum Gene Ther 2006; 17: 882–889.
Vaughan EE, Dean DA . Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther 2006; 13: 422–428.
Mesika A, Kiss V, Brumfeld V, Ghosh G, Reich Z . Enhanced intracellular mobility and nuclear accumulation of DNA plasmids associated with a karyophilic protein. Hum Gene Ther 2005; 16: 200–208.
Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS . Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem 2000; 275: 1625–1629.
Bulinski JC, Gundersen GG . Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays 1991; 13: 285–293.
Hammond JW, Cai D, Verhey KJ . Tubulin modifications and their cellular functions. Curr Opin Cell Biol 2008; 20: 71–76.
Li R, Gundersen GG . Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9: 860–873.
Vaughan EE, Geiger RC, Miller AM, Loh-Marley PL, Suzuki T, Miyata N et al. Microtubule acetylation through HDAC6 inhibition results in increased transfection efficiency. Mol Ther 2008; 16: 1841–1847.
Geiger RC, Taylor W, Glucksberg MR, Dean DA . Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Therapy 2006; 13: 725–731.
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 2006; 16: 2166–2172.
Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci 2007; 27: 3571–3583.
Barlow AL, van Drunen CM, Johnson CA, Tweedie S, Bird A, Turner BM . dSIR2 and dHDAC6: two novel, inhibitor-resistant deacetylases in Drosophila melanogaster. Exp Cell Res 2001; 265: 90–103.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002; 417: 455–458.
Verdel A, Curtet S, Brocard MP, Rousseaux S, Lemercier C, Yoshida M et al. Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr Biol 2000; 10: 747–749.
Kelkar SA, Pfister KK, Crystal RG, Leopold PL . Cytoplasmic dynein mediates adenovirus binding to microtubules. J Virol 2004; 78: 10122–10132.
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP . The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003; 115: 727–738.
Badding MA, Vaughan EE, Dean DA . Transcription factor plasmid binding modulates microtubule interactions and intracellular trafficking during gene transfer. Gene Therapy 2011; 19: 338–346.
Dean DA, Dean BS, Muller S, Smith LC . Sequence requirements for plasmid nuclear import. Exp Cell Res 1999; 253: 713–722.
Wilson GL, Dean BS, Wang G, Dean DA . Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J Biol Chem 1999; 274: 22025–22032.
Dean DA . Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res 1997; 230: 293–302.
Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E et al. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther 2000; 11: 151–165.
Wang Z, Sheetz MP . One-dimensional diffusion on microtubules of particles coated with cytoplasmic dynein and immunoglobulins. Cell Struct Funct 1999; 24: 373–383.
Mallik R, Petrov D, Lex SA, King SJ, Gross SP . Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol 2005; 15: 2075–2085.
Kaufman CD, Geiger RC, Dean DA . Electroporation- and mechanical ventilation-mediated gene transfer to the lung. Gene Therapy 2010; 17: 1098–1104.
Taylor W, Gokay KE, Capaccio C, Davis E, Glucksberg M, Dean DA . The effects of cyclic stretch on gene transfer in alveolar epithelial cells. Mol Ther 2003; 7: 542–549.
King SJ, Schroer TA . Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol 2000; 2: 20–24.
Paschal BM, Shpetner HS, Vallee RB . MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol 1987; 105: 1273–1282.
Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR . Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science 2005; 308: 1469–1472.
Morris RL, Hollenbeck PJ . Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol 1995; 131: 1315–1326.
Li D, Sewer MB . RhoA and DIAPH1 mediate adrenocorticotropin-stimulated cortisol biosynthesis by regulating mitochondrial trafficking. Endocrinology 2010; 151: 4313–4323.
Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL et al. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 2008; 135: 1098–1107.
Suh J, Wirtz D, Hanes J . Efficient active transport of gene nanocarriers to the cell nucleus. Proc Natl Acad Sci USA 2003; 100: 3878–3882.
de Bruin K, Ruthardt N, von Gersdorff K, Bausinger R, Wagner E, Ogris M et al. Cellular dynamics of EGF receptor-targeted synthetic viruses. Mol Ther 2007; 15: 1297–1305.
Welte MA . Bidirectional transport along microtubules. Curr Biol 2004; 14: R525–R537.
Posta F, D'Orsogna MR, Chou T . Enhancement of cargo processivity by cooperating molecular motors. Phys Chem Chem Phys 2009; 11: 4851–4860.
Eldib M, Dean DA . Cyclic stretch of alveolar epithelial cells alters cytoskeletal micromechanics. Biotechnol Bioeng 2011; 108: 446–453.
Dunn S, Morrison EE, Liverpool TB, Molina-Paris C, Cross RA, Alonso MC et al. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci 2008; 121: 1085–1095.
Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ . Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell 2010; 21: 572–583.
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 2002; 21: 6820–6831.
Desai A, Mitchison TJ . Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 1997; 13: 83–117.
Westermann S, Weber K . Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol 2003; 4: 938–947.
Regnard C, Audebert S, Boucher D, Larcher JC, Edde B, Denoulet P . Microtubules: functional polymorphisms of tubulin and associated proteins (structural and motor MAP's). C R Seances Soc Biol Fil 1996; 190: 255–268.
Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N . Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci 1992; 103 Pt 4 953–964.
Saragoni L, Hernandez P, Maccioni RB . Differential association of tau with subsets of microtubules containing posttranslationally-modified tubulin variants in neuroblastoma cells. Neurochem Res 2000; 25: 59–70.
Xie P . Mechanism of processive movement of monomeric and dimeric kinesin molecules. Int J Biol Sci 2010; 6: 665–674.
Kikkawa M . The role of microtubules in processive kinesin movement. Trends Cell Biol 2008; 18: 128–135.
Krebs A, Goldie KN, Hoenger A . Complex formation with kinesin motor domains affects the structure of microtubules. J Mol Biol 2004; 335: 139–153.
Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Therapy 1999; 6: 482–497.
Barry ME, Pinto-Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA . Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum Gene Ther 1999; 10: 2461–2480.
Dean DA . Peptide nucleic acids: versatile tools for gene therapy strategies. Adv Drug Deliv Rev 2000; 44: 81–95.
Gasiorowski JZ, Dean DA . Postmitotic nuclear retention of episomal plasmids is altered by DNA labeling and detection methods. Mol Ther 2005; 12: 460–467.
Piperno G, Fuller MT . Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 1985; 101: 2085–2094.
Rogers SS, Waigh TA, Zhao X, Lu JR . Precise particle tracking against a complicated background: polynomial fitting with Gaussian weight. Phys Biol 2007; 4: 220–227.
Acknowledgements
We would like to thank Rui Zhou, Erin Vaughan, Mootaz Eldib and Michael Welte for their insightful discussions and technical advice. This work was supported in part by a predoctoral fellowship from the Founders Affiliate of the American Heart Association (MB), and grants EB9903 and ES01247 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Badding, M., Dean, D. Highly acetylated tubulin permits enhanced interactions with and trafficking of plasmids along microtubules. Gene Ther 20, 616–624 (2013). https://doi.org/10.1038/gt.2012.77
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/gt.2012.77