Abstract
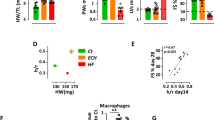
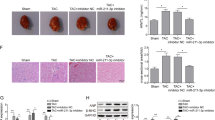
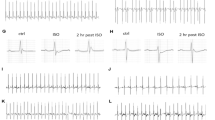
Altered alpha- and beta-adrenergic receptor signaling is associated with cardiac hypertrophy and failure. Stromal cell-derived factor-1α (SDF-1α) and its cognate receptor CXCR4 have been reported to mediate cardioprotection after injury through the mobilization of stem cells into injured tissue. However, little is known regarding whether SDF-1/CXCR4 induces acute protection following pathological hypertrophy and if so, by what molecular mechanism. We have previously reported that CXCR4 physically interacts with the beta-2 adrenergic receptor and modulates its downstream signaling. Here we have shown that CXCR4 expression prevents beta-adrenergic receptor-induced hypertrophy. Cardiac beta-adrenergic receptors were stimulated with the implantation of a subcutaneous osmotic pump administrating isoproterenol and CXCR4 expression was selectively abrogated in cardiomyocytes using Cre-loxP-mediated gene recombination. CXCR4 knockout mice showed worsened fractional shortening and ejection fraction. CXCR4 ablation increased susceptibility to isoproterenol-induced heart failure, by upregulating apoptotic markers and reducing mitochondrial function; cardiac function decreases whereas fibrosis increases. In addition, CXCR4 expression was rescued with the use of cardiotropic adeno-associated viral-9 vectors. CXCR4 gene transfer reduced cardiac apoptotic signaling, improved mitochondrial function and resulted in a recovered cardiac function. Our results represent the first evidence that SDF-1/CXCR4 signaling mediates acute cardioprotection through modulating beta-adrenergic receptor signaling in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tissier R, Berdeaux A, Ghaleh B, Couvreur N, Krieg T, Cohen MV et al. Making the heart resistant to infarction: how can we further decrease infarct size? Front Biosci 2008; 13: 284–301.
Yellon DM, Baxter GF . Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: distant dream or near reality? Heart 2000; 83: 381–387.
Carden DL, Granger DN . Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190: 255–266.
Katholi RE, Couri DM . Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens 2011; 2011: 495349.
El-Armouche A, Zolk O, Rau T, Eschenhagen T . Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res 2003; 60: 478–487.
Zierhut W, Zimmer HG . Significance of myocardial alpha- and beta-adrenoceptors in catecholamine-induced cardiac hypertrophy. Circ Res 1989; 65: 1417–1425.
Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ . Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 1993; 87: 454–463.
Hadcock JR, Ros M, Watkins DC, Malbon CC . Cross-regulation between G-protein-mediated pathways. Stimulation of adenylyl cyclase increases expression of the inhibitory G-protein, Gi alpha 2. J Biol Chem 1990; 265: 14784–14790.
Zhao M, Fajardo G, Urashima T, Spin JM, Poorfarahani S, Rajagopalan V et al. Cardiac pressure overload hypertrophy is differentially regulated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol 2010; 301: H1461–H1470.
Port JD, Bristow MR . Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J M Cell Cardiol 2001; 33: 887–905.
Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci 2001; 938: 36–45; discussion 45-37.
Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation 2004; 109: 2454–2461.
Onuffer JJ, Horuk R . Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci 2002; 23: 459–467.
Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY . Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol 2004; 483: 175–186.
Insel PA, Tang CM, Hahntow I, Michel MC . Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim Biophys Acta 2007; 1768: 994–1005.
Busillo JM, Benovic JL . Regulation of CXCR4 signaling. Biochim Biophys Acta 2007; 1768: 952–963.
LaRocca TJ, Schwarzkopf M, Altman P, Zhang S, Gupta A, Gomes I et al. beta2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol 2010; 56: 548–559.
Lou Q, Janardhan A, Efimov IR . Remodeling of calcium handling in human heart failure. Adv Exp Med Biol 2012; 740: 1145–1174.
Larocca TJ, Jeong D, Kohlbrenner E, Lee A, Chen J, Hajjar RJ et al. CXCR4 gene transfer prevents pressure overload induced heart failure. J Mol Cell Cardiol 2012; 53: 223–232.
Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T et al. G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation 2012; 126: 1972–1982.
Pan J, Fukuda K, Kodama H, Sano M, Takahashi T, Makino S et al. Involvement of gp130-mediated signaling in pressure overload-induced activation of the JAK/STAT pathway in rodent heart. Heart Vessels 1998; 13: 199–208.
Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A et al. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol 2000; 151: 117–130.
Anderson ME . CaMKII and a failing strategy for growth in heart. J Clin Invest 2009; 119: 1082–1085.
Serneri GG, Modesti PA, Boddi M, Cecioni I, Paniccia R, Coppo M et al. Cardiac growth factors in human hypertrophy. Relations with myocardial contractility and wall stress. Circ Res 1999; 85: 57–67.
Baker KM, Chernin MI, Wixson SK, Aceto JF . Renin-angiotensin system involvement in pressure-overload cardiac hypertrophy in rats. Am J Physiol 1990; 259: H324–H332.
Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 2009; 15: 171–181.
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR . Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393: 595–599.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382: 635–638.
Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA 1998; 95: 9448–9453.
Agarwal U, Ghalayini W, Dong F, Weber K, Zou YR, Rabbany SY et al. Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circ Res 2010; 107: 667–676.
Berenji K, Drazner MH, Rothermel BA, Hill JA . Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol 2005; 289: H8–H16.
Schiaffino S, Samuel JL, Sassoon D, Lompre AM, Garner I, Marotte F et al. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload—induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res 1989; 64: 937–948.
Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 2005; 280: 35760–35766.
Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP et al. Beta-arrestin- but not G protein-mediated signaling by the "decoy" receptor CXCR7. Proc Natl Acad Sci USA 2010; 107: 628–632.
Eom TY, Roth KA, Jope RS . Neural precursor cells are protected from apoptosis induced by trophic factor withdrawal or genotoxic stress by inhibitors of glycogen synthase kinase 3. J Biol Chem 2007; 282: 22856–22864.
Rimbaud S, Garnier A, Ventura-Clapier R . Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep 2009; 61: 131–138.
Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A et al. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab 2012; 15: 324–335.
Kaya Y, Cebi A, Soylemez N, Demir H, Alp HH, Bakan E . Correlations between oxidative DNA damage, oxidative stress and coenzyme Q10 in patients with coronary artery disease. Int J Med Sci 2012; 9: 621–626.
Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS . Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation 2012; 126: 314–324.
Entman ML, Smith CW . Postreperfusion inflammation: a model for reaction to injury in cardiovascular disease. Cardiovasc Res 1994; 28: 1301–1311.
Hunter JJ, Chien KR . Signaling pathways for cardiac hypertrophy and failure. N Engl J Med 1999; 341: 1276–1283.
Gaasch WH . Left ventricular radius to wall thickness ratio. Am J Cardiol 1979; 43: 1189–1194.
Hudlicka O, Brown M, Egginton S . Angiogenesis in skeletal and cardiac muscle. Physiol Rev 1992; 72: 369–417.
Hoeks J, van Baak MA, Hesselink MK, Hul GB, Vidal H, Saris WH et al. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am J Physiol Endocrinol Metab 2003; 285: E775–E782.
Beurel E, Jope RS . The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 2006; 79: 173–189.
Gomez-Sintes R, Hernandez F, Lucas JJ, Avila J . GSK-3 mouse models to study neuronal apoptosis and neurodegeneration. Front Mol Neurosci 2011; 4: 45.
Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J et al. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem 1991; 266: 24613–24620.
Petrich BG, Molkentin JD, Wang Y . Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J 2003; 17: 749–751.
Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR . The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med 2004; 200: 1145–1156.
Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD . Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 1997; 100: 169–179.
Tarzami ST, Calderon TM, Deguzman A, Lopez L, Kitsis RN, Berman JW . MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a G(alphai)-independent pathway. Biochem Biophys Res Commun 2005; 335: 1008–1016.
Acknowledgements
This study was supported in part by the American Heart Association (grant GRNT4180006 to STT) and the National Institutes of Health (grant K02HL102163-01 to STT). We would like to thank Drs Roger Hajjar and Antoine Chaanine for their helpful advice and suggestions throughout the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Wang, E., Jarrah, A., Benard, L. et al. Deletion of CXCR4 in cardiomyocytes exacerbates cardiac dysfunction following isoproterenol administration. Gene Ther 21, 496–506 (2014). https://doi.org/10.1038/gt.2014.23
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/gt.2014.23
This article is cited by
-
LncRNA TINCR improves cardiac hypertrophy by regulating the miR-211-3p-VEGFB-SDF-1α-CXCR4 pathway
Laboratory Investigation (2022)
-
PET Tracers for Imaging Cardiac Function in Cardio-oncology
Current Cardiology Reports (2022)