Abstract
Intraspecific hybrid zones involving long-lived woody species are rare and can provide insights into the genetic basis of early-diverging traits in speciation. Within the landscape-dominant Hawaiian tree, Metrosideros polymorpha, are morphologically distinct successional varieties, incana and glaberrima, that dominate new and old lava flows, respectively, below 1200 me on volcanically active Hawai‘i Island, with var. glaberrima also extending to higher elevations and bogs. Here, we use morphological measurements on 86 adult trees to document the presence of an incana–glaberrima hybrid zone on the 1855 Mauna Loa lava flow on east Hawai‘i Island and parent–offspring analysis of 1311 greenhouse seedlings from 71 crosses involving 72 adults to estimate heritabilities and genetic correlations among vegetative traits. Both the variation in adult leaf pubescence at the site and the consistency between adult and offspring phenotypes suggest the presence of two hybrid classes, F1s and var. incana backcrosses, as would be expected on a relatively young lava flow. Nine nuclear microsatellite loci failed to distinguish parental and hybrid genotypes. All four leaf traits examined showed an additive genetic basis with moderate to strong heritabilities, and genetic correlations were stronger for the more range-restricted var. incana. The differences between varieties in trait values, heritabilities and genetic correlations, coupled with high genetic variation within but low genetic variation between varieties, are consistent with a multi-million-year history of alternating periods of disruptive selection in contrasting environments and admixture in ephemeral hybrid zones. Finally, the contrasting genetic architectures suggest different evolutionary trajectories of leaf traits in these forms.
Similar content being viewed by others
Introduction
Hybrid zones—‘narrow regions in which genetically distinct populations meet, mate, and produce hybrids’ (Barton and Hewitt, 1985)—are particularly fruitful settings for studies of speciation (Harrison, 1990; Hewitt, 2001). Intraspecific hybrid zones in particular are relatively few in number, yet the parapatric ‘races’ that form them represent important stages of the speciation process (Hewitt, 1988), allowing studies of gene flow, selection and the evolution of isolating barriers at the early stages of speciation (Barton and Hewitt, 1985). Unfortunately, the resolution of hybrid zone studies involving very closely related taxa is often limited by uncertain designation of hybrid genotypes because of weak genetic differentiation between parental forms (Nielsen et al., 2003).
Within species, genetic architecture can be shaped by selection operating on key adaptive traits, and it contributes significantly to the evolutionary trajectories of complex phenotypes (Sinervo and Svensson, 2002; Hansen, 2006). For example, taxa with broad ecological niches (for example, generalists) may have greater phenotypic and genetic variation and lower genetic correlations than specialists in which selection has favored stronger genetic correlations among key traits (Grant and Grant, 1994; Sinervo and Svensson, 2002). Within hybrid zones the genetic architectures of hybridizing taxa can be altered depending on the extent of admixture. Although introgression will generally increase genetic variation within hybridizing taxa (Grant and Grant, 1994; Seehausen, 2004), it is less clear how genetic correlations between traits will be altered through hybridization. For taxa with contrasting genetic correlational structures, introgression is expected to weaken genetic correlations within taxa (Grant and Grant, 1994). Overall, hybridization can increase population evolvability through elevated genetic variation and altered genetic architectures (Hansen, 2006) while facilitating ecological diversification through the generation of novel combinations of adaptive traits (Grant and Grant, 1994; Seehausen, 2004).
Metrosideros polymorpha Gaud. (‘ohi’a lehua or ‘ohi’a) is a landscape-dominant tree species in Hawaii that offers the opportunity to examine the genetic architecture of key phenotypic traits at the early stages of ecological divergence in trees. This hypervariable species comprises both pubescent and glabrous forms that are differentially distributed across elevation and rainfall gradients on the Hawaiian Islands (Dawson and Stemmermann, 1990; Kitayama et al., 1997). Four of Hawai‘i Island’s five named varieties share the same common chloroplast haplotype (Percy et al., 2008) and show significant within-taxon cohesion across the island and isolation from each other at neutral genetic loci, suggesting M. polymorpha as an unusual case of incipient radiation in trees (Stacy et al., 2014). All forms have hermaphrodite red (or less often orange or yellow), shaving-brush-shaped flowers arranged in showy inflorescences, but differ from each other in vegetative traits (Dawson and Stemmermann, 1990). Common garden studies reveal heritable differences among varieties (or among populations sampled across environmental gradients) in morphological (Corn and Hiesey, 1973), leaf anatomical and physiological (Stemmermann, 1983; Kitayama et al., 1997; Cordell et al., 1998, 2000) traits, including leaf pubescence (Stemmermann, 1983; Kitayama et al., 1997). Whereas these studies indicate heritability of the traits that are used to distinguish varieties of M. polymorpha on Hawai‘i Island, heritabilities within varieties are unknown.
The two most abundant varieties of ‘ohi’a on Hawai‘i Island, early-successional var. incana and late-successional var. glaberrima, are ecotypes (Turesson, 1922) that dominate new and old lava flows, respectively, anywhere moisture is sufficient below roughly 1200 m above sea level (Mueller-Dombois, 1983; Stemmermann, 1983; Mueller-Dombois, 1987; Kitayama et al., 1997). Var. glaberrima is unique among Hawai‘i Island varieties in its ecological breadth, ranging from low-elevation stands through to ~1500 m in elevation with scattered individuals occurring higher. Var. glaberrima occurs also in bogs on the oldest volcano, Kohala, and hosts the most neutral genetic variation of any variety on the island (Stacy et al., 2014). Replacement of early-successional var. incana by late-successional var. glaberrima on an aging lava flow occurs over a period of >1400 but <3000 years, depending on lava type and elevation (Drake and Mueller-Dombois, 1993; Drake, 1993; Kitayama et al., 1997). The forms can be distinguished entirely through leaf characters; the appressed (abaxial) pubescent leaves of var. incana tend to be smaller than the glabrous leaves of var. glaberrima (Dawson and Stemmermann, 1990). Previous studies of vars. incana and glaberrima on Hawai‘i Island documented different leaf nitrogen contents (Vitousek et al., 1995), and the differential adaptation of their seedlings to light and nitrogen (Morrison and Stacy, 2014), both of which vary significantly between new and old substrates on east Hawai‘i Island (Crews et al., 1995). Other studies of glabrous and pubescent seedlings or trees from lower elevations (indicative of these two varieties) grown in a common garden revealed differences in water retention (Stemmermann, 1983), cuticle thickness and possibly osmotic potentials and photosynthesis rates (Kitayama et al., 1997). The direction of these differences is consistent with specialization of var. incana to the especially harsh abiotic conditions (including drought; Kitayama et al., 1997) of new lava flows or otherwise dry areas at lower elevations versus the more generalist nature of var. glaberrima. Despite these functional differences, var. incana and glaberrima are the most weakly genetically differentiated pair of ‘ohi’a varieties on Hawai‘i Island (mean population pairwise FST=0.05 (P<0.01) versus FST=0.056–0.151 for other pairwise combinations of varieties; DeBoer and Stacy, 2013; Stacy et al., 2014).
On the 1855 Mauna Loa lava flow below ∼1200 m in elevation, adults of both successional varieties are present as well as trees with intermediate phenotypes, suggesting extensive intraspecific hybridization on this intermediate-aged lava flow (Kitayama et al., 1997); above that elevation on the same flow, hybridization with the high-elevation var. polymorpha is apparent. To test the hypothesis that there is an intraspecific hybrid zone on the 1855 Mauna Loa lava flow on east Hawai‘i Island, we analyzed the vegetative morphology and microsatellite genotypes of 86 trees at ~880 m elevation. We used a classic crossing design and parent–offspring analysis to resolve the parental and hybrid status of individuals in the hybrid zone based on vegetative traits. Finally, we used this same crossing design to test the hypothesis that the heritabilities of, and genetic correlations among, key leaf traits differ between the two successional varieties representing contrasting niche breadths.
Materials and methods
Study site
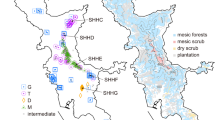
This study centered on the population of M. polymorpha occurring within a ~1200 by 200 m area at ~880 m above sea level on the 1855 Mauna Loa lava flow near Saddle Road, East Hawai‘i Island (Figure 1). This intermediate-aged (~150 years old at the time of the study) lava flow supports a mixed population of both vars. incana and glaberrima and morphologically intermediate trees, and the low population density at the site promotes flowering of many trees of unusually small stature (Supplementary Figure S1). These features allowed canopy access and crossing among forms that would be difficult or impossible elsewhere on the island, though restricted access to the canopies of tall (early-successional) var. incana trees led to fewer crosses involving this taxon. M. polymorpha is the sole tree species at the site.
(top) Map of East Hawai‘i Island showing the study site (oversized hatched circle) and (bottom) the locations of the 86 trees included in the analysis of morphology. Recent lava flows from Mauna Loa are indicated by shading, with the 1855 flow (study site) shown in black. The light gray background indicates lava flows >3000 years old. The map of the study trees is split in two halves, corresponding roughly with mile markers (MM) 11–11.5 and MM 11.5–12 on Saddle Road (dashed line); the tree circled with the dashed line is common to both halves. Adults were designated as var. incana (I), var. glaberrima (G) or an incana–glaberrima hybrid (H) based on leaf pubescence (see text). The underlined symbols indicate trees not included in the hand-crossing study (n=14).
Morphological characterization of adults
Eighty-six trees (>1.3 m tall with reproductive structures; Figure 1) representing four tree classes (two varieties and two hybrid classes) were haphazardly chosen from among trees at the site producing ample floral buds and/or flowers during May–July 2006 and 2007. The 86 trees were well dispersed (mean±s.d. intertree distance=329±223 m; range=1.4–1054 m). Nine vegetative characters were measured on each tree (leaf width, leaf length, petiole length, internode length, leaf area, leaf mass, plant height, plant width and number of stems) from which three composite traits were computed (leaf shape, specific leaf area and plant shape); one index of herbivory was also recorded. Trees were sorted to type based on pubescence on the abaxial surface of mature nonsenescing leaves such that permanent leaf pubescence=var. incana (I); glabrous (pubescence absent)=var. glaberrima (G); and leaf pubescence removable by rubbing (caducous)=incana-glaberrima hybrids (H). Hybrids were further split into two groups based on the persistence of pubescence: class-1 hybrids (hereafter H1): pubescence is removed easily, and class-2 hybrids (hereafter H2): pubescence is more resistant to rubbing. Classification of all trees by this method was fully consistent across project personnel. On East Hawai‘i Island, the morphotypes designated as hybrids are very rare outside of intermediate-aged lava flows (all authors, personal observation). With the exception of some adults of early-successional var. incana, which on average exceeded 4.5 m in height and are presumably the oldest trees on the lava flow, the canopies of trees were accessible (2.46±0.11 m tall; Supplementary Figure S2). Leaf measurements were done for each of 10 well-dispersed, mature but not senescing leaves (third node from new growth) from each tree and averaged. Internode lengths were averaged across five mature internodes (second internode from growing tip) per tree, and specific leaf area was estimated using 20 leaves per tree following the formula: specific leaf area=leaf area in cm2 per dry mass. Leaf area was determined with a LI-COR LI-3100 Leaf Area Meter (Lincoln, NE, USA), and dry mass calculations were taken on leaves following 48 h at 80 °C. The intensity of infestation (herbivory) by gall-forming psyllid fly larva (gall load) was also scored for each tree on a scale of 0 (no galls) to 3 (intense galling).
All response variables were examined for normality and equal variances across tree types, and were normalized as necessary using Johnson’s transformation in Minitab 16 (Minitab Inc., Pennsylvania, PA, USA). The Johnson’s transformation algorithm, similar to the Box–Cox algorithm, yields the function that transforms the data to best fit the normal distribution (Minitab Inc.; Johnson, 1949; Chou et al., 1998). To characterize adult morphology, a principal components analysis (PCA) was done for all 86 trees on 7 traits showing pairwise Pearson’s correlations <0.8 (Supplementary Table S1), and analyses of variance (ANOVAs) with Tukey’s multiple comparisons (P<0.05 family error rate) were done to compare tree types for each of the three significant principal component axes. Follow-up one-way ANOVAs were done for all individual traits, followed by Tukey’s post hoc pairwise comparisons to test for differences in vegetative morphology among tree types. The exceptions were internode length, number of stems and gall load that were resistant to transformation and thus analyzed using nonparametric Kruskal–Wallis tests; significant tests were followed by post hoc Mann–Whitney U-tests to identify pairwise differences between tree types.
Genetic structure within the hybrid zone
Two leaf buds were collected from each of the 86 trees and held at −80 °C until DNA extractions could be done. Frozen leaf material was homogenized with Lysing Matrix A tubes in the FastPrep-24 Instrument for 40 s at 4.0 m s−1 (MP Biomedicals, Santa Ana, CA, USA). DNA was extracted from the lysate using a QIAGEN DNeasy Plant Mini Kit (QIAGEN, Valencia, CA, USA) following the manufacturer’s protocol with the following modifications: extension of cell lysis incubation time to 15 min, one extra centrifuging to completely dry the membrane after washing and a decrease in the elution buffer to 75 μl. Extracted DNA was precipitated with 100% ethanol and 3 M Na-acetate (pH 5.2), vacuum dried, washed with 70% ethanol and resuspended in elution buffer (QIAGEN). Nine previously published microsatellite loci were amplified: MePo501, MePo503, MePo506, MePo508, MePo511, MePo512, MePo513, MePo514 and MePo515 (Crawford et al., 2008). PCR was performed in a 10 μl volume (1 × GoTaq Flexi buffer, 2 mM MgCl2, 0.5 μM each dNTP, 1.25 U Go Taq DNA polymerase (Promega, Madison, WI, USA), 0.4 μM of a dye-labeled forward primer and an unlabeled reverse primer and 1–10 ng DNA) following Crawford et al. (2008) with the exceptions of an initial annealing temperature of 55 °C and a final extension step at 72 °C for 10 min. PCR products were separated in 1.5% agarose gels, visualized by SYBR Safe DNA gel stain (Invitrogen, Carlsbad, CA, USA) and sized on a Beckman-Coulter (Fullerton, CA, USA) CEQ8000. Genotypes were determined using CEQ 8000 Software, version 8.0 (Beckman-Coulter).
The microsatellite data from the hybrid zone were supplemented with data from seven and four adult populations of vars. incana and glaberrima, respectively, occurring throughout Hawai‘i Island (Stacy et al., 2014). Allelic richness measures for the two varieties and both hybrid populations (H1 and H2) were calculated using ADZE (Szpiech et al., 2008) in two ways: (1) using all populations of vars. incana and glaberrima from across the island (n=138 and 94 individuals, respectively) and (2) using just the populations of these varieties occurring within the hybrid zone (using n=13 individuals per population for rarefaction in both cases). Homozygosity by loci was also calculated analogously using Cernicalin (Aparicio et al., 2006). One-way ANOVA was used to compare each of allelic richness, private allelic richness and homozygosity among island-wide var. incana, island-wide glaberrima and hybrids. MICRO-CHECKER (Oosterhout et al., 2004) revealed significant null allele frequencies at three loci. The number of clusters among var. incana, var. glaberrima and their hybrids (K) was estimated using STRUCTURE 2.3.4 with the Bayesian Markov chain Monte Carlo approach (Pritchard et al., 2000), assuming admixture and correlated allele frequencies and allowing for the presence of null alleles at three loci. A burn-in period of 10 000 steps followed by 100 000 Monte Carlo replicates were simulated with K=1 through 20, and the optimal number of clusters was evaluated using an ad hoc statistic (ΔK; Evanno et al., 2005). Finally, the proportion of each individual belonging to parental or hybrid classes was evaluated using NewHybrids (Anderson and Thompson, 2002), using noninformative Jeffrey priors and 100 000 iterations.
Controlled crosses
Hand pollinations were done using 72 of the 86 hybrid zone trees, involving all possible combinations of tree types for analyses of adult fertility and cross-fertility (reported elsewhere; Stacy et al., unpublished data); phenotypes of 1311, 2.5-year-old offspring from these crosses were used in the current study to estimate the genetic basis and heritabilities of the traits used to distinguish the two parental varieties. Each cross involved a single pair of trees crossed reciprocally such that each partner acted as both pollen donor and maternal tree (for example, Tree 3→Tree 5 and Tree 5→Tree 3). Seventy-one pairs of trees were crossed. Within each tree type, selection of individual trees for hand pollinations was haphazard from among all flowering trees of that type. Seedlings from these crosses were used in three analyses, each involving a subset of independent crosses (that is, such that each tree within the data set is used in a single cross only). First, a subset of 20 crosses involving only pure vars. glaberrima and incana adults was examined to uncover the genetic basis of the phenotypic differences between varieties, including additive vs dominant genetic elements, and to aid the identification of F1 trees at the site. Second, 33 independent crosses involving all four phenotypes were used in a quantitative genetic analysis of the traits used to distinguish these varieties. Finally, 15 crosses involving only var. glaberrima adults were used to estimate genetic variation within varieties; inferences were also drawn from the equivalent analyses with the four pure var. incana crosses. Each outcross involved pollen from a single donor transferred to ⩾20 flowers on a maternal tree, spread across two or more inflorescences. Flowers receiving pollen were emasculated at the stamen-emergence stage of anthesis and covered with mesh bags. Pollinations were done 2–3 days after emasculation when stigmas become receptive; Stacy et al., unpublished data), and flowers that were asynchronous with the majority were removed. Pollen was transferred directly from anthers to stigma until the stigma was saturated, and pollinator exclusion bags were left on the experimental inflorescences for 14 days after pollination.
Upon maturation (~7.5 months later) fruits were collected and stored in open coin envelopes in an air-conditioned lab for 2 weeks to ensure capsule dehiscence. Seeds from mature experimental fruits (⩽10 per cross) were sown (one fruit per 2, 5 × 10 cm wells) atop well-draining media covered with a thin layer of black sand and kept for 4 weeks in a misthouse under low light (∼600 μmol m−2 s−1) and 20 s of mist every 30 min during daylight hours) and then transferred to a greenhouse (overhead water 3 × daily with amounts adjusted as needed). At ∼2 months post germination, seedlings were thinned to a maximum of six well-spaced seedlings per 5 × 10 cm well, and trays were rotated within the greenhouse monthly. At ∼8 months after germination, a subset from each family (mean=13) was transferred from communal wells to individual 10 cm pots. A six-sided dice was used to randomly select two seedlings out of the six from each well as needed for potting up. Phenotyping of seedlings was done at ~2.5 years of age when adult leaf characters had clearly emerged. Seedlings were measured or scored for eight vegetative traits (leaf length and width, petiole length, number of stems, abaxial and adaxial leaf pubescence, stem pubescence and stem color); leaf shape was then calculated as leaf length/leaf width. Length measurements were taken with dial calipers and averaged across two mature leaves, and pubescence was scored from 0 (glabrous) to 2 (permanently pubescent). Stem color was scored 1, 2 or 3 for green (light), red and purple (dark), respectively. Internode length was initially measured, but dismissed because of high variation within individual seedlings.
Genetic basis of vegetative traits
To determine the genetic basis of traits used to distinguish vars. incana and glaberrima and to aid the identification of F1 trees in the field, a subset of 20 independent within- and between-variety crosses was extracted from the full set of crosses (that is, excluding any crosses involving purported hybrid trees). One-way ANOVAs were done to compare each of the interval-scale morphological traits across the three seedling classes (within-var. glaberrima, within-var. incana and between-variety crosses; normal distributions and equal variances were confirmed), and analogous Kruskal–Wallis tests and post hoc pairwise Mann–Whitney U-tests were done for rank-order measures.
Heritability and genetic correlation estimation through parent–offspring analysis
To estimate heritability of variety-diagnostic traits, traits were measured in adults in the field and their offspring in the greenhouse for three overlapping sets of crosses: 630 offspring from 33 independent (selected via coin toss), reciprocal crosses involving 66 randomly paired adults of all phenotypes (with seedlings from reciprocal crosses pooled within families; mean=19.1 seedlings/family, range: 8–35); 298 offspring from 15 independent, reciprocal crosses involving 30 randomly paired adults of var. glaberrima (with reciprocal crosses pooled; mean=19.9 seedlings/family, range: 16–30); and offspring from 4 independent, reciprocal crosses involving 8 randomly paired adults of var. incana (with reciprocal crosses pooled; mean=14 seedlings/family, range: 9–19). The following steps were taken for each subset of crosses separately. Pearson’s pairwise correlation tests were run between maternal trees and pollen donors for all interval-scale morphological measures, and Spearman’s rank correlations were run for all pairwise comparisons involving either abaxial pubescence or number of stems (both rank order). For each of four leaf traits (leaf length, leaf width, leaf shape and petiole length), narrow-sense heritability was calculated using weighted (by family size) linear regression analysis of mean offspring values against mid-parent values (Falconer and Mackay, 1996). Maternal and paternal heritabilities were calculated correcting for correlations between maternal trees and pollen donors as: h2=2b/(1+r), where b is the parent–offspring correlation coefficient and r is the correlation between maternal trees and pollen donors; and maternal effect sizes were calculated by subtracting paternal hn2 from maternal hn2 for each leaf trait (Falconer and Mackay, 1996). The s.e. for each single-parent heritability estimate was obtained by doubling the s.e. for the regression coefficient from the weighted regression of the offspring values on the parental values. For all rank-order measures, Spearman’s correlations were run to compare traits both among seedlings and between seedlings and adults. Pairwise genetic correlations among leaf length, leaf width and petiole length were calculated following:  , where cov is the parent–offspring covariance, and i and j are traits one and two, respectively (Falconer and Mackay, 1996).
, where cov is the parent–offspring covariance, and i and j are traits one and two, respectively (Falconer and Mackay, 1996).
Results
Morphological characterization of adults
Pairwise correlations among the 12 morphological traits ranged from −0.359 (number of stems and plant height) to 0.935 (leaf mass and leaf area; Supplementary Table S1). Analysis of adult morphology of 86 trees in the hybrid zone revealed differences among all four tree classes defined by pubescence type. PCA of adult morphological traits yielded three significant axes (eigenvalues >1) that in combination accounted for a total of 64.4% (33.7, 16.3 and 14.4%, respectively) of the variation across the 86 trees measured (Supplementary Table S2 and Supplementary Figure S3). PC1 was loaded most heavily with leaf area, petiole length and number of stems and captured the range of plant forms from typically large-leaved multi-stemmed var. glaberrima to more tree-like, smaller-leaved var. incana with hybrids in between. With hybrids pooled, PC1 differentiated all three tree classes (F2, 83=33.97, P<0.001, R2=43.68; Figure 2a). The ANOVA of PC1 scores with hybrids split into two classes showed significant differentiation between the two hybrid classes, but not between var. glaberrima and H1 (F3, 82=30.11, P<0.001, R2=50.68%; Figure 2b). PC2 scores (Johnson transformed; weighted most heavily with internode length) varied significantly (with hybrids pooled: F2, 83=4.02, P=0.022) or nearly significantly (with hybrids separate: F3, 82=2.72, P=0.05) between hybrids (H1) and var. incana trees (Figure 2). PC2 scores of hybrids were lower than those of the two parental varieties, rather than intermediate. PC3 scores did not vary across the tree types.
Examination of individual trait variation revealed significant differences between trees of vars. glaberrima and incana at 9 of 12 vegetative traits as well as gall load (Table 1). The two varieties were largely differentiated by leaf dimensions alone (Supplementary Figure S4) in addition to leaf pubescence. The pooled hybrids were intermediate between the two varieties and differed from both varieties in leaf length, width, area and mass (Supplementary Figure S5). Divided, the two hybrid classes defined by leaf pubescence characters differed from each other at 5 of the 13 morphological traits (including gall load; Figure 3 and Supplementary Figure S6).
Mean values±1 s.e. of 6 leaf traits of 86 trees in an intraspecific M. polymorpha hybrid zone. Tree types are as in Figure 2. Shared letters indicate no significant difference at α=0.05.
Genetic structure within the hybrid zone
Allelic richness estimates per 13 individuals averaged across loci were significantly higher for var. glaberrima (6.17±0.31 (s.d.)) than for var. incana (5.61±0.21) sampled from across the island with hybrids in between (mean of pooled hybrids: 6.12±0.086; F2, 12=9.54, P=0.003; Supplementary Table S3). In comparison with populations of the two varieties occurring just within the hybrid zone, allelic richness of H1 (6.03±1.04) and H2 (6.20±1.04) were again intermediate between those of var. incana (5.68±1.01) and var. glaberrima (6.52±1.01); private allelic richness was lower for var. incana relative to the other tree classes both island wide (F2, 12=8.70, P=0.005) and locally (Supplementary Table S3). Homozygosity by locus did not differ across tree classes, ranging from 0.36±0.01 to 0.39±0.04 (P=0.96). The STRUCTURE analysis with the H1 and H2 hybrid populations and eight and five populations of vars. incana and glaberrima, respectively, from throughout the island revealed k=2 as the most likely number of clusters, but only modest differentiation between varieties and no demarcation of hybrid populations (Supplementary Figures S7 and S8). The NewHybrids analysis failed to classify parental varieties or hybrids correctly, designating a majority of trees in the F2 class; notably, however, ‘pure incana’ made up a significantly greater proportion of trees than did ‘pure glaberrima’ (Supplementary Figure S9).
Genetic basis of vegetative traits
The analysis of phenotypes of 2.5-year-old seedlings derived from 20 independent within- and between-variety crosses involving only pure-variety parents revealed significant differences between the varieties with F1 hybrids intermediate but slightly closer to var. glaberrima. Var. glaberrima, F1 and var. incana seedlings differed in abaxial leaf pubescence scores (H=16.11, d.f.=2, P<0.001; Figure 4a) and stem pubescence scores (Supplementary Figure S10). Adaxial leaf pubescence and stem color also differed between var. incana and var. glaberrima seedlings, with F1 seedlings intermediate but not unique (that is, not significantly different from both parental varieties), and stem number trended lower for F1s compared with pure-variety seedlings, although the difference was significant only in comparison with pure incana seedlings (F2, 17=4.99, P=0.02; Supplementary Figure S10). Examination of the key variety-diagnostic trait—abaxial pubescence—at the individual seedling level revealed variation within families and cross types. Of 298 seedlings derived from var. glaberrima × var. glaberrima crosses, 280 were fully glabrous as expected; 18 showed nonpersistent pubescence, and none possessed the dense, permanent pubescence characteristic of var. incana. Of 56 var. incana × var. incana seedlings, 45 showed dense, persistent abaxial leaf pubescence as expected, 6 showed nonpersistent pubescence and 5 were glabrous. Of 125 F1 seedlings, 54 showed intermediate leaf pubescence as expected, 54 were glabrous and 17 showed heavier pubescence than expected (though none showed dense, persistent pubescence). F1 seedlings with unexpected leaf pubescence appeared in even frequencies in the two reciprocal cross types. Overall, there was a slight shift toward glabrousness in the seedling pool.
Individual value plots of abaxial (bottom) leaf pubescence of parents and offspring (family-level means) from (a) 20 independent crosses involving pure-variety trees only (shared superscripts indicate no significant difference at α=0.05), and (b) the same from 42 not fully independent crosses involving pure-variety and H1 trees only (for graph only). Tree types are as in Figure 2. Solid bars indicate medians.
Expanding the analysis to include H1 adults (purported F1s) in the pool of crosses yielded purported backcross glaberrima and backcross incana seedlings with phenotypes largely intermediate between those of F1 seedlings and vars. glaberrima and incana seedlings, respectively (Figure 4b and Supplementary Table S4). The consistency of phenotypes of the purported backcross incana seedlings, including leaf pubescence, with those of H2 adults at the study site suggests that the latter are backcross incana trees.
Heritability estimation through parent–offspring analysis
Mid-parent–offspring narrow-sense heritabilities were estimated for the four leaf traits that are sometimes used in addition to leaf pubescence to distinguish vars. glaberrima and incana. The analysis of 33 random independent crosses involving all tree types revealed moderate, statistically significant heritabilities for all four traits (Table 2 and Figure 5). Although not used for estimation of heritabilities, four other rank-order traits recorded for these seedlings also showed highly significant correlations with mid-parent values of abaxial leaf pubescence (Supplementary Figure S11), consistent with a heritable basis for each. Analysis of the subset of 15 independent within-var. glaberrima crosses revealed generally higher heritability values, though with higher s.e. values because of the lower sample size (Table 2 and Figure 6). Finally, analysis of the four independent within-var. incana crosses revealed high heritability estimates (leaf length: 0.552±0.427, P=0.325; leaf width: 1±0.048, P=0.002; leaf shape: >1±>1, P=0.191; and petiole length: 1±0.118, P=0.017; not shown). Maternal effect sizes ranged from 0.075 (petiole length) to 0.285 (leaf width) within var. glaberrima and were lower for all three traits for the 33 crosses involving all tree types (Table 2). Direct estimates of maternal effects within var. incana were not possible because of the limited number of within-variety crosses.
Scatter plots of mid-parent (x axis) and mid-offspring (y axis) values for (a) leaf length, (b) leaf width, (c) leaf shape (leaf length/leaf width) and (d) petiole length from 33 independent crosses involving 66 randomly paired adults of all phenotypes at the study site. Weighted linear regressions: leaf length: F1, 31=18.85, P<0.001, R2=35.81; leaf width: F1, 31=5.68, P=0.023, R2=12.76; leaf shape: F1, 31=21.17, P<0.001, R2=38.66; petiole length: F1, 31=17.60, P<0.001, R2=34.15.
Scatter plots of mid-parent (x axis) and mid-offspring (y axis) values for (a) leaf length, (b) leaf width, (c) leaf shape (leaf length/leaf width) and (d) petiole length from 15 independent crosses involving 30 randomly paired adults of var. glaberrima at the study site. Linear regressions: leaf length: F1, 13=5.49, P=0.036, R2=24.26; leaf width: F1, 13=3.27, P=0.094, R2=13.93; leaf shape: F1, 13=18.4, P=0.001, R2=54.47; petiole length: F1, 13=5.16, P=0.041, R2=22.91.
Genetic correlations
Pairwise genetic correlations were calculated among leaf length, leaf width and petiole length for each of the three subsets of crosses described above. Within var. incana, leaf length and width were very strongly, positively correlated (r~1.0±0.004 (s.e.)), and both traits were negatively correlated with petiole length (leaf length: −0.778±0.079; leaf width: −0.627±0.028). In contrast, within var. glaberrima, leaf length and width were decoupled (r=−0.104±0.152), and both leaf traits were positively correlated with petiole length (leaf length: 0.746±0.005; leaf width 0.432±0.009). Genetic correlations among the three traits for the full set of 33 crosses involving all tree types were intermediate to the values calculated for the two parental varieties separately (leaf length × leaf width: 0.643±0.036; leaf length × petiole length: 0.187±0.057; leaf width × petiole length: 0.087±0.077).
Discussion
This study verifies an intraspecific hybrid zone between two common and weakly genetically diverged varieties of the hypervariable, landscape-dominant tree, M. polymorpha, on Hawai‘i Island. Furthermore, through comparison of phenotypes of hybrid zone adults and offspring derived from controlled crosses among them, we posit a model for hybrid zone genotypes and confirm heritabilities of the traits used to distinguish these varieties. We also show stronger genetic correlations among leaf traits in the early-successional var. incana, as predicted given its narrower ecological niche, and intermediate genetic correlations in the hybrid zone, consistent with hybridization.
The different plant shapes observed for successional vars. incana and glaberrima in this study can be added to a growing list of phenotypic and ecological differences that have been observed between these varieties on Hawai‘i Island, including leaf morphology, leaf nitrogen content, water retention, cuticle thickness, seedling-stage responses to light and soil nitrogen and seed germination responses to light and heat (Stemmermann, 1983; Dawson and Stemmermann, 1990; Drake, 1993; Vitousek et al., 1995; Kitayama et al., 1997; Morrison and Stacy, 2014). In addition to the presence–absence of leaf pubescence that distinguishes these forms, all measures of leaf size and shape, as well as plant shape, recorded for adults in the hybrid zone also differed between these forms; only specific leaf area, internode length and plant width did not differ. At the study site, pubescent, early-successional var. incana trees were typically single stemmed with smaller, rounder leaves, whereas glabrous, late-successional var. glaberrima trees were single- or multi-stemmed with larger, longer-petioled and often longer leaves.
Intraspecific hybrid zones on intermediate-aged lava flows
The analysis of 12 morphological characters of 86 trees at the study site indicates the presence of an incana–glaberrima hybrid zone at 880 m above sea level on the 1855 Mauna Loa lava flow on east Hawai‘i Island. In fact, the abundance of morphologically intermediate trees on other intermediate-aged lava flows on east Hawai‘i Island (all authors, personal observation) suggests that incana–glaberrima hybrid zones form readily on intermediate-aged substrates at low–middle elevations on the island. Aging lava flows represent an intermediate, transitional environment between fresh lava fields and mature rainforests where proximity of trees of the two varieties is increased (Howard et al., 1997), the lack of a stable ecological community promotes hybrid community formation (Moore, 1977) and growth and survivorship of intervarietal hybrids may be at least as high as those of the parental varieties in their home environments (Anderson, 1948; Muller, 1952). The high rainfall at low–middle elevations (Giambelluca et al., 2013) should facilitate succession and coexistence of the two varieties and their hybrids (Kitayama et al., 1997). Indeed, the abundance of hybrids at the study site suggests that growth and survivorship of hybrids are high in these intermediate environments and further suggests that prezygotic barriers between the successional varieties in sympatry are weak. The high abundance of hybrids on intermediate-aged lava flows, coupled with immigrant inviability of vars. glaberrima and incana on new and old lava flows respectively (Morrison and Stacy, 2014), suggests that these ephemeral incana–glaberrima hybrid zones likely best fit the ‘geographically bounded hybrid superiority model’ (Moore, 1977; Hamilton and Aitken, 2013). This model would predict that growth and survivorship of hybrids in both parental environments are low, a conclusion that is supported by the low frequency of apparent hybrids outside of intermediate-aged lava flows. This observation, coupled with the paucity of pubescent trees in mature rainforests and glabrous trees on fresh lava flows below ∼1200 m in elevation, suggests strong purifying selection on both varieties and their hybrids during the transition from seedlings to adulthood in parental habitats. Strong selection on juvenile stages appears to be an important determinant of adult distribution in trees (Petit and Hampe, 2006; Poorter, 2007).
With their recent common ancestry, sympatry and presumed extended history of alternating cycles of introgression and separation on the chronosequence of active volcanoes that make up the island chain, vars. incana and glaberrima form hybrid zones that likely capture elements of both primary and secondary hybrid zones (Hewitt, 1988). It is not clear from available data whether the initial divergence of these forms, which appears to have occurred on an older island (Stacy et al., 2014), occurred through selection in parapatry or if allopatry was involved. Nonetheless, a history of persistent, close association is suggested by their current sympatric or parapatric distributions on at least five main Hawaiian Islands (Lana‘i, not observed), whose substrates range in age from 0 years (Hawai‘i Island) to 5 million years (Kaua‘i). Vars. incana and glaberrima appear to fit Endler's (1977) model of (incipient) parapatric speciation wherein a continuously distributed population diverges across a heterogeneous environment, albeit a dynamic environment of active and extinct volcanoes.
Whereas a majority of hybrid zones are thought to be long lived, resulting from climate changes such as the glacial–interglacial transition in northern temperate zones (Barton and Hewitt, 1985; Hewitt, 1988), the incana–glaberrima hybrid zones on volcanically active Hawai‘i Island are ephemeral. An adult population of var. incana may appear on a new lava flow by roughly 27 years after cooling (Drake and Mueller-Dombois, 1993), and var. glaberrima adults may appear as early as 50 years after cooling (Kitayama et al., 1995). Complete replacement of var. incana by var. glaberrima takes >1400 but <3000 years, depending on lava type and elevation (Drake and Mueller-Dombois, 1993; Kitayama et al., 1997). Thus, pubescent and glabrous trees, including hybrids, may coexist on an aging lava flow for roughly 1400 to 2400 years. Given the fertility of hybrids (this study), and assuming a roughly 50-year generation time, this scenario would indicate very roughly 30–50 generations of introgression between varieties with each new lava flow. As conditions on a lava flow progress to the late-seral stage, replacement of trees with pubescent leaves ceases, leaving a monotypic stand of var. glaberrima (Drake and Mueller-Dombois, 1993) introgressed with var. incana alleles. This conclusion is consistent with the lower proportion of ‘pure glaberrima’ than ‘pure incana’ fractions in the NewHybrids analysis of these varieties across Hawai‘i Island (this study) and the relatively greater allelic richness and private allelic richness observed for var. glaberrima than for var. incana, both in the hybrid zone and across Hawai‘i Island (Stacy et al., 2014; and this study).
Analysis of adult morphology at the study site suggests the presence of at least two classes of hybrids on the 1855 Mauna Loa flow on Hawai‘i Island. Both hybrid classes are identifiable by their intermediate leaf pubescence, as was also observed in hybrids between glabrous and pubescent species of Asian oaks (Wei et al., 2015). The relative abundances of these forms at the site and the known pattern of forest succession, coupled with the results of our parent–offspring analysis, suggest that H1 and H2 trees are F1s and backcrosses to early-successional var. incana, respectively. The ease with which F1 seeds were produced in this study is consistent with the high abundance of purported F1 trees at the study site, and the mean and median leaf pubescence scores (and means of other phenotypic traits) of F1 offspring produced through reciprocal hand-crosses between the varieties were consistent with those of purported F1 trees in the field. The production of backcross var. incana trees through frequent crossing between F1s and var. incana would be expected given the dominance of early-successional var. incana on young lava flows and the propensity of F1 hybrids to mate with the more abundant parental taxon (Lepais et al., 2009). The mean/median leaf pubescence scores of offspring produced through reciprocal hand-crosses between purported F1s and var. incana were consistent with those used to designate the purported backcross incana (H2) trees in the field. In fact, all 12 of the morphological traits measured on H2 adults in the hybrid zone showed values that were intermediate between those of purported F1 and var. incana, as expected for backcross var. incana trees (assuming quantitative traits with an additive genetic basis). The preponderance of F1 and backcross incana trees on the 1855 lava flow is fully consistent with the composition of genotypes that would be expected on a young lava flow (~150 years old) where mixing of tree varieties has occurred for only a few generations. The exceptionally weak differentiation between these varieties severely limited the utility of the SSR data for distinguishing hybrid genotypes (Nielsen et al., 2003; Vaha and Primmer, 2006), and neither measures of allelic richness nor heterozygosity (inferred from homozygosity estimates) were greater for the hybrid populations than for the parental taxa sampled on either island-wide or local scales. Both of these results are consistent with a prolonged history of recurring introgression between these varieties.
Inheritance of variety-diagnostic traits
The principal character used to distinguish vars. incana and glaberrima—the presence or complete absence of permanent abaxial leaf pubescence—is heritable (Stemmermann, 1983) and appears to have an additive genetic basis with a partial dominance of the glabrous condition. In fact, leaf morphology generally showed a slight dominance of traits from one of the parental taxa (var. glaberrima), as was also observed for F1 offspring between two Asian oak species (Wei et al., 2015). The majority (94 and 80%) of seedlings produced through within-var. glaberrima and within-var. incana crosses were fully glabrous and permanently pubescent, respectively, as expected if pubescence is heritable. Only 43% of seedlings produced through between-variety crosses, however, showed the predicted intermediate pubescence, with another 43% of the seedlings being glabrous. Importantly, of these three cross types, seedlings with the dense, permanent pubescence diagnostic of var. incana were produced only through within-var. incana crosses (these seedlings were also produced in crosses between H2 trees and between H2 and var. incana trees). The additive genetic basis with a partial dominance of the glabrous condition was apparent even though the wild nature of the study trees and their long generation times prohibited diallel crosses between homogeneous lines to determine the genetic basis of leaf pubescence or any other trait (see, for example, Zaiter et al., 1990; Van Dam et al., 1999).
Variation in leaf pubescence and a tendency toward glabrousness also occur in open-pollinated seedlings of M. polymorpha. In a common garden, whereas seedlings from wild glabrous maternal trees tended to be glabrous, those from wild pubescent trees were highly variable, consistent with an overall tendency toward glabrousness, and seedling phenotypes from both classes of maternal trees on younger substrates (that is, the 1855 lava flow, the site of the current study) were highly variable (Kitayama et al., 1997). The distribution of leaf pubescence scores among open-pollinated seedlings from glabrous and pubescent maternal trees in the study by Kitayama et al. (1997) appears fully consistent with both historical introgression and current hybridization between varieties where maternal trees are surrounded by adults of the opposite variety (that is, glabrous trees on young lava flows and pubescent trees on old). The variation in seedling pubescence characters in both studies leads to some uncertainty in our initial designations of var. glaberrima and hybrid individuals at the study site based on leaf pubescence alone. However, these designations are also supported by several vegetative traits of those adults and their offspring in addition to leaf pubescence. Misclassification of hybrid trees as var. glaberrima would only lead to underestimation of the differences between the varieties, suggesting that these estimates may be conservative.
The parent–offspring analysis of hybrid zone adults and their offspring revealed moderate to strong heritabilities for the other traits that are also used to distinguish vars. incana and glaberrima. The range of values observed within the hybrid zone, h2=0.27 for leaf width to h2=0.73 for leaf shape, are consistent with (if slightly higher than) those generally reported for plant morphological traits (mean h2=0.26±0.001; reviewed by Geber and Griffen, 2003). Even within var. glaberrima, heritabilities were substantial (h2=0.31 to h2=0.85). This might be unexpected, given the anticipated significance of leaf traits for fitness (Nicotra et al., 2011) and the contrasting directional selection imposed on juveniles of these forms in their parental habitats (Fisher, 1930). The maintenance of heritable variation within varieties may be explained by their history of recurring, prolonged episodes of introgression on intermediate-aged lava flows that may facilitate adaptation to changing environments (Grant and Grant, 1994; Seehausen, 2004). The modest heritabilities observed here for leaf traits suggest that phenotypic plasticity and possibly nonadditive genetic variation (that is, residual variation; Houle, 1992) may also be important in shaping the leaf characters of these varieties. Finally, slight maternal effects were detected in leaf traits that may help to ensure adaptation of seedlings to their local environment (Roach and Wulff, 1987).
Genetic architecture of leaf traits
Vars. incana and glaberrima differ in the genetic architecture of simple leaf traits that may reflect their contrasting home environments (Nicotra et al., 2011) as well as differences in the varieties’ ecological breadths. The contrasts in leaf shape between these varieties may reflect tradeoffs between structure and function experienced in early- and late-seral forests. For example, the greater petiole length and leaf length-to-width ratio of var. glaberrima should permit greater light capture per unit leaf area in the wet forest understory through a larger separation of leaf area from stems (Takenaka, 1994). In contrast, the more or less fixed leaf shape of var. incana may be the optimal leaf shape for the high-light, water-limited conditions of new lava flows. Whereas leaf length and width were strongly genetically correlated for early-successional var. incana, these traits were decoupled for late-successional var. glaberrima. The correlation between petiole length and leaf size (length and width) also differed, being positive in var. glaberrima and negative in var. incana. The stronger genetic correlation among leaf traits in var. incana may reflect the narrower range of conditions under which this variety is found on Hawai‘i Island (young lava flows at low to middle elevations or otherwise drier areas). Var. glaberrima, in contrast, is unique among Hawai‘i Island varieties in its ecological breadth, ranging from low-elevation stands to beyond 1500 m in elevation, including bogs on the oldest volcano, Kohala. Moreover, because of its broader range, it co-occurs and hybridizes with each of the three other varieties of M. polymorpha on the island (early-successional var. incana, high-elevation var. polymorpha and riparian var. newellii; Stacy et al., 2014). Ongoing introgression with forms of such contrasting leaf morphologies may also contribute to the weak genetic correlations within var. glaberrima (Grant and Grant, 1994). Finally, the lack of a genetic correlation between leaf length and width for var. glaberrima suggests that leaf shape in this variety should be more flexible in response to selection (Hansen, 2006). This conclusion is consistent with the purported evolution of the stenophyllous (that is, narrow)-leaved, riparian var. newellii from var. glaberrima on Hawai‘i Island within the past 500 000 years (Stacy et al., 2014).
Summary
This study confirms the presence of an intraspecific hybrid zone on the 1855 lava flow at 880 m elevation on Hawai‘i Island between the common successional vars. incana (early) and glaberrima (late) of the landscape-dominant tree species, M. polymorpha. Results further suggest the presence of two abundant hybrid genotypes, F1 and backcross incana trees, as would be expected on a lava flow where mixing of varieties has occurred for only a few generations. The strong genetic basis underlying several vegetative traits that are used to distinguish vars. incana and glaberrima coupled with the weak neutral genetic differentiation between these varieties are consistent with strong disruptive selection for these traits and an extended history of recurring episodes of introgression on intermediate-aged lava flows. The contrasting heritabilities and genetic correlational structures for leaf traits in the two varieties in spite of recurring introgression suggest different genetic architectures, and thus evolutionary trajectories, of simple leaf traits in these forms. The incana–glaberrima hybrid zones should be particularly useful for studies of reproductive isolating barriers at the early stages of ecological speciation in trees and the genetic architecture of such barriers. These results highlight the value of hypervariable tree species for insights into genetic architecture at the early stages of ecological divergence in trees.
Data archiving
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.6f785.
References
Anderson EC, Thompson EA . (2002). A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160: 1217–1229.
Anderson E . (1948). Hybridization of the habitat. Evolution 2: 1–9.
Aparicio JM, Ortego J, Cordero PJ . (2006). What should we weigh to estimate heterozygosity, alleles or loci? Mol Ecol 15: 4659–4665.
Barton NH, Hewitt G . (1985). Analysis of hybrid zones. Ann Rev Ecol Syst 16: 113–148.
Chou Y, Polansky AM, Mason RL . (1998). Transforming non-normal data to normality in statistical process control. J Qual Tech 30: 133–141.
Cordell S, Goldstein G, Melcher P, Meinzer F . (2000). Photosynthesis and freezing avoidance in ohia (Metrosideros polymorpha at treeline in Hawaii. Arct Antarct Alp Res 32: 381–387.
Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek P . (1998). Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113: 188–196.
Corn CA, Hiesey WM . (1973). Altitudinal variation in Hawaiian Metrosideros. Am J Bot 60: 991–1002.
Crawford NG, Hagen C, Sahli HF, Stacy EA, Glenn TC . (2008). Permanent genetic resources: fifteen polymorphic microsatellite DNA loci from Hawaii's Metrosideros polymorpha (Myrtaceae: Myrtales), a model species for ecology and evolution. Mol Ecol Resour 8: 308–310.
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D et al. (1995). Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76: 1407–1424.
Dawson J, Stemmermann L . (1990) Metrosideros (Gaud) In: Wagner WL, Herbst DR, Sohmer SH (eds) Manual of the Flowering Plants of Hawai’i. Univ. Hawai’i Press: Honolulu, HI. pp 964–970.
DeBoer N, Stacy EA . (2013). Divergence within and among 3 varieties of the endemic tree, ‘Ōhi’a Lehua (Metrosideros polymorpha on the eastern slope of Hawai’i Island. J Hered 104: 449–458.
Drake DR . (1993). Germination requirements of Metrosideros polymorpha, the dominant tree of Hawaiian lava flows and rain forests. Biotropica 25: 461–467.
Drake DR, Mueller-Dombois D . (1993). Population development of rain forest trees on a chronosequence of Hawaiian lava flows. Ecology 74: 1012–1019.
Endler JA . (1977) Geographic Variation, Speciation, and Clines. Princeton Univ. Press: Princeton, NJ.
Evanno G, Regnaut S, Goudet J . (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620.
Falconer DS, Mackay TFC . (1996) Introduction to Quantitative Genetics, 4th edn. Longman: Essex, UK.
Fisher RA . (1930) The Genetical Theory of Natural Selection. Clarendon Press: Oxford, UK.
Geber MA, Griffen LR . (2003). Inheritance and natural selection on functional traits. Int J Plant Sci 164: S21–S42.
Giambelluca TW, Chen Q, Frazier AG, Price JP, Chen Y, Chu P et al. (2013). Online rainfall atlas of Hawai'i. Bull Am Meteorol Soc 94: 313–316.
Grant PR, Grant BR . (1994). Phenotypic and genetic effects of hybridization in Darwin's finches. Evolution 48: 297–316.
Hamilton JA, Aitken SN . (2013). Genetic and morphological structure of a spruce hybrid (Picea sitchensis X P. glauca zone along a climatic gradient. Am J Bot 100: 1651–1662.
Hansen TF . (2006). The evolution of genetic architecture. Ann Rev Ecol Evol Syst 37: 123–157.
Harrison RG . (1990). Hybrid zones: windows on evolutionary process. Oxford Surv Evol Biol 7: 69–128.
Hewitt GM . (1988). Hybrid zones-natural laboratories for evolutionary studies. Trends Ecol Evol 3: 158–167.
Hewitt GM . (2001). Speciation, hybrid zones and phylogeography - or seeing genes in space and time. Mol Ecol 10: 537–549.
Houle D . (1992). Comparing evolvability and variability of quantitative traits. Genetics 130: 195–204.
Howard DJ, Preszler RW, Williams J, Fenchel S, Boecklen WJ . (1997). How discrete are oak species? Insights from a hybrid zone between Quercus grisea and Quercus gambelii. Evolution 51: 747–755.
Johnson NL . (1949). Systems of frequency curves generated by methods of translation. Biometrika 36: 149–176.
Kitayama K, Pattison R, Cordell S, Webb D, Mueller-Dombois D . (1997). Ecological and genetic implications of foliar polymorphism in Metrosideros polymorpha Gaud. (Myrtaceae) in a habitat matrix on Mauna Loa, Hawaii. Ann Bot 80: 491–497.
Kitayama K, Mueller-Dombois D, Vitousek PM . (1995). Primary succession of Hawaiian montane rain forest on a chronosequence of eight lava flows. J Veg Sci 6: 211–222.
Lepais O, Petit R, Guichoux E, Lavabre J, Alberto F, Kremer A et al. (2009). Species relative abundance and direction of introgression in oaks. Mol Ecol 18: 2228–2242.
Moore WS . (1977). An evaluation of narrow hybrid zones in vertebrates. Quart Rev Biol 52: 263–277.
Morrison KR, Stacy EA . (2014). Intraspecific divergence and evolution of a life-history trade-off along a successional gradient in Hawaii's Metrosideros polymorpha. J Evol Biol 27: 1192–1204.
Mueller-Dombois D . (1987). Forest dynamics in Hawaii. Trends Ecol Evol 2: 216–220.
Mueller-Dombois D . (1983). Canopy dieback and successional processes in Pacific forests. Pac Sci 37: 317–325.
Muller CH . (1952). Ecological control of hybridization in Quercus: a factor in the mechanism of evolution. Evolution 1: 147–161.
Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL et al. (2011). The evolution and functional significance of leaf shape in the angiosperms. Func Plant Biol 38: 535–552.
Nielsen EE, Hansen MM, Ruzzante DE, Meldrup D, Grønkjær P . (2003). Evidence of a hybrid‐zone in Atlantic cod (Gadus morhua in the Baltic and the Danish Belt Sea revealed by individual admixture analysis. Mol Ecol 12: 1497–1508.
Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P . (2004). MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4: 535–538.
Percy DM, Garver AM, Wagner WL, James HF, Cunningham CW, Miller SE et al. (2008). Progressive island colonization and ancient origin of Hawaiian Metrosideros (Myrtaceae). Proc R Soc B 275: 1479–1490.
Petit RJ, Hampe A . (2006). Some evolutionary consequences of being a tree. Annu Rev Ecol Evol Syst 37: 187–214.
Poorter L . (2007). Are species adapted to their regeneration niche, adult niche, or both? Am Nat 169: 433–442.
Pritchard JK, Stephens M, Donnelly P . (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945–959.
Roach DA, Wulff RD . (1987). Maternal effects in plants. Annu Rev Ecol Syst 18: 209–235.
Seehausen O . (2004). Hybridization and adaptive radiation. Trends Ecol Evol 19: 198–207.
Sinervo B, Svensson E . (2002). Correlational selection and the evolution of genomic architecture. Heredity 89: 329–338.
Stacy EA, Johansen JB, Sakishima T, Price DD, Pillon Y . (2014). Incipient radiation within the dominant Hawaiian tree Metrosideros polymorpha. Heredity 113: 334–342.
Stemmermann L . (1983). Ecological studies of Hawaiian Metrosideros in a successional context. Pac Sci 37: 361–373.
Szpiech ZA, Jakobsson M, Rosenberg NA . (2008). ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24: 2498–2504.
Takenaka A . (1994). Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol Res 9: 109–114.
Turesson G . (1922). The species and the variety as ecological units. Hereditas 3: 100–113.
Vaha J, Primmer CR . (2006). Efficiency of model‐based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci. Mol Ecol 15: 63–72.
Van Dam N, Hare J, Elle E . (1999). Inheritance and distribution of trichome phenotypes in Datura wrightii. J Hered 90: 220–227.
Vitousek PM, Turner DR, Kitayama K . (1995). Foliar nutrients during long-term soil development in Hawaiian montane rain forest. Ecology 76: 712–720.
Wei L, Li Y, Zhang H, Liao W . (2015). Variation in morphological traits in a recent hybrid zone between closely related Quercus liaotungensis and Q. mongolica (Fagaceae). J Plant Ecol 8: 224–229.
Zaiter HZ, Coyne DP, Steadman JR, Beaver JS . (1990). Inheritance of abaxial leaf pubescence in beans. J Am Soc Hort Sci 115: 158–160.
Acknowledgements
We thank Hawai‘i Island DOFAW for permission to work in state forest; Kainana Francisco, Jan Iyo, Melissa Johnson, Ala Leka, Katherine Miller, Bhama Paritosh and Richard Short for assistance in the field or greenhouse; and UH Hilo’s CAFNRM for use of facilities at Pana’ewa Farm. Patty Moriyasu provided advice on seedling care, Anne Veillet and Heather Sahli assisted with genotyping and Rebecca Ostertag provided lab equipment. Funding was provided by NSF 0542350 and NSF 0954274 to EAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Heredity website
Supplementary information
Rights and permissions
About this article
Cite this article
Stacy, E., Johansen, J., Sakishima, T. et al. Genetic analysis of an ephemeral intraspecific hybrid zone in the hypervariable tree, Metrosideros polymorpha, on Hawai‘i Island. Heredity 117, 173–183 (2016). https://doi.org/10.1038/hdy.2016.40
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/hdy.2016.40
This article is cited by
-
A framework for establishing a rapid ‘Ōhi‘a death resistance program
New Forests (2023)