Abstract
The purpose of this study was to determine serum α2-HS glycoprotein (AHSG) concentration and its diagnostic accuracy in preeclampsia. In this case–control study, the serum C-reactive protein (CRP) and AHSG levels were measured in 93 preeclamptic patients and in 127 healthy pregnant women by immunoturbidimetry and radial immunodiffusion. The serum CRP levels were significantly higher, whereas the serum AHSG concentrations were significantly lower in the preeclamptic group than in the control group (median (25th to 75th percentile), CRP: 6.71 mg l−1 (2.76–12.69) vs. 3.38 mg l−1 (1.69–7.27), respectively; AHSG: 660 μg ml−1 (612–768) vs. 744 μg ml−1 (660–816), respectively; P<0.001 for both). In preeclamptic patients, the serum AHSG concentrations showed significant inverse correlations with systolic blood pressure and serum CRP levels. A low serum AHSG level (⩽720 μg ml−1) was significantly associated with preeclampsia (adjusted odds ratio (95% confidence interval): 3.69 (1.82–7.51); P<0.001). According to the receiver operating characteristic curves, the measurement of serum AHSG concentrations was as accurate as that of serum CRP levels to detect preeclampsia. In conclusion, serum AHSG concentration is decreased and reflects—at least partly—systemic inflammation in preeclampsia.
Similar content being viewed by others
Introduction
Preeclampsia is a severe complication of human pregnancy, with a worldwide incidence of 2–10%.1 It is one of the leading causes of maternal, as well as perinatal morbidity and mortality, even in developed countries.2 Despite intensive research efforts, the etiology and pathogenesis of preeclampsia are not completely understood. Increasing evidence suggests that an excessive maternal systemic inflammatory response to pregnancy, which is supposed to be triggered by inflammatory stimuli derived from the hypoxic and oxidatively stressed placenta, has a crucial role in the pathogenesis of the disease.3, 4 The systemic inflammatory response involves a rise in the number and activation of leukocytes (monocytes, granulocytes and natural killer cells) with the production of proteases and proinflammatory cytokines leading to T-helper type 1 bias, as well as the activation of endothelial cells, platelets, the coagulation and complement systems and the production of acute-phase proteins.5, 6, 7, 8 The development of preeclampsia is influenced by both genetic and environmental risk factors, suggesting its multifactorial inheritance.9, 10, 11, 12, 13, 14
The α2-Heremans–Schmid (α2-HS) glycoprotein (fetuin-A, AHSG), the human homolog of bovine fetuin, is an abundant plasma/serum protein synthesized predominantly by hepatocytes.15 It belongs to the cystatin superfamily of cysteine proteinase inhibitors. AHSG is one of the rare negative acute-phase proteins, the synthesis of which is decreased in the liver during the acute-phase response.16 The protein has diverse biological functions, including the regulation of osteogenesis and bone resorption, the prevention of unwanted mineralization and the inhibition of insulin receptor autophosphorylation and tyrosine kinase activity.17, 18 In addition, it promotes phagocytosis and has opsonic properties.19, 20 It is interesting that the tissue expression and blood levels of AHSG are particularly high in the fetus, and it has been postulated that the protein participates in the development of tissues, such as fetuin in other species.21 However, its exact significance is still obscure.
We have recently reported that the serum AHSG concentration is decreased in patients with the syndrome of hemolysis, elevated liver enzymes and a low platelet count (the HELLP syndrome).22 The aim of this study was to determine the serum AHSG concentration and its diagnostic accuracy in preeclampsia without the HELLP syndrome. Given that the maternal syndrome of preeclampsia is characterized by a generalized intravascular inflammatory reaction with an acute-phase response and that AHSG acts as a negative acute-phase protein, we hypothesized that the serum AHSG concentration is decreased in preeclampsia.
Methods
Study participants
Ninety-three preeclamptic patients and 127 normotensive (blood pressure<140 mm Hg systolic and <90 mm Hg diastolic), healthy pregnant women with uncomplicated pregnancies were involved in this case–control study. The study participants were enrolled in the 1st Department of Obstetrics and Gynecology and in the Department of Obstetrics and Gynecology of the Kútvölgyi Clinical Center, Semmelweis University, Budapest, Hungary. All women were Caucasian and resided in the same geographical area in Hungary. Exclusion criteria were multifetal gestation, chronic hypertension, diabetes mellitus, autoimmune disease, angiopathy, renal disorder, maternal or fetal infection and fetal congenital anomaly. The women were fasting, none were in active labor and none had rupture of membranes.
Preeclampsia was defined by an increased blood pressure (⩾140 mm Hg systolic or ⩾90 mm Hg diastolic on ⩾2 occasions at least 6 h apart) that occurred after 20 weeks of gestation in a woman with previously normal blood pressure, accompanied by proteinuria (⩾0.3 g per 24 h).23 Blood pressure returned to normal by 12 weeks postpartum in each preeclamptic study patient. Pregnant women with the HELLP syndrome were not enrolled in this study. Fetal growth restriction was diagnosed when the fetal birth weight was below 10th percentile for gestational age and gender, based on the Hungarian birth weight percentiles.24
The study protocol was approved by the Regional and Institutional Committee of Science and Research Ethics of the Semmelweis University, and a written informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki.
Biological samples
Maternal blood samples were obtained from an antecubital vein into native tubes and centrifuged at room temperature with a relative centrifugal force of 3000 g for 10 min. The aliquots of serum were stored at −80 °C until the analyses were performed.
Laboratory methods
The serum C-reactive protein (CRP) concentration was measured by particle-enhanced immunoturbidimetric assay (Cobas Integra 800; Roche, Mannheim, Germany; catalog no. 20764930). Human CRP agglutinated with latex particles coated with monoclonal anti-CRP antibodies. The precipitate was determined turbidimetrically at 552 nm. The detection limit was 0.07 mg l−1, whereas the intra/interassay variabilities at mean values of 6.2 and 142 mg l−1 were 1.8, 2.9, 1.5 and 2.7%, respectively.
The serum concentration of AHSG was determined by the radial immunodiffusion method using Goat Anti-Human α2-HS Glycoprotein IgG fraction (DiaSorin Inc., Stillwater, MN, USA; catalog no. 81931). Pooled plasma of healthy blood donors was used for calculating the standard values. The intra/interassay variabilities were 3.6 and 6.2%, respectively.
Statistical analysis
The normality of continuous variables was assessed using the Shapiro–Wilk W-test. As the continuous variables were not normally distributed, non-parametric statistical methods (the Mann–Whitney U-test and the Spearman rank order correlation) were used. The Fisher's exact and Pearson χ2 tests were carried out to compare categorical variables between groups. As the serum levels of AHSG showed a skewed distribution, we performed analysis of covariance with logarithmically transformed data. The diagnostic accuracy of serum AHSG measurements was evaluated using the receiver operating characteristic curve analysis. Multivariate logistic regression was carried out with adjustments for maternal age, body mass index (BMI) and gestational age at blood draw.
Statistical analyses were performed applying the following software: STATISTICA (version 8.0; StatSoft Inc., Tulsa, OK, USA), Statistical Package for the Social Sciences (version 15.0 for Windows; SPSS Inc., Chicago, IL, USA) and MedCalc for Windows (version 10.0.1.0; MedCalc Software, Mariakerke, Belgium). For all statistical analyses, P<0.05 was considered statistically significant.
In the article, data are reported as median (interquartile range) for continuous variables and as number (percent) for categorical variables.
Results
Patient characteristics
The clinical characteristics of the study participants are shown in Table 1. There were no statistically significant differences between the two study groups in terms of maternal age and the percentage of smokers and primiparas. The BMI and gestational age at blood draw were significantly higher in the preeclamptic group than in the control group. The systolic and diastolic blood pressures were significantly higher, whereas the gestational age at delivery and the fetal birth weight were significantly lower in the preeclamptic group compared with those in the control group. Fetal growth restriction was absent in control individuals, whereas the frequency of this condition was 22.6% in the preeclamptic group.
Laboratory parameters
As presented in Table 1 and Figure 1, serum CRP levels were significantly higher, whereas serum AHSG concentrations were significantly lower in preeclamptic patients than in normotensive, healthy pregnant women. The difference in the serum AHSG levels between the two study groups remained significant even after adjustments for maternal age, BMI and gestational age at blood draw in analysis of covariance.
In the group of preeclamptic patients, no statistically significant differences were observed in serum CRP and AHSG levels between preeclamptic patients with and without fetal growth restriction (median (25th to 75th percentile), CRP: 7.30 mg l−1 (2.80–14.24) vs. 6.53 mg l−1 (2.74–12.69); AHSG: 636 μg ml−1 (574–720) vs. 660 μg ml−1 (624–780)).
Relationship of clinical characteristics and serum CRP levels with serum AHSG concentrations in preeclampsia
We investigated whether the clinical characteristics and serum CRP levels of preeclamptic patients were related to serum AHSG concentrations by calculating the Spearman rank order correlation coefficients (continuous variables) and by the Mann–Whitney U-test (categorical variables). In preeclamptic patients, serum AHSG concentrations showed significant inverse correlations with systolic blood pressure (Spearman R=−0.23; P<0.05) and serum CRP levels (Spearman R=−0.21; P<0.05). However, no other relationship was found between clinical features (maternal age, smoking status, parity, BMI and gestational age at blood draw, diastolic blood pressure, gestational age at delivery and fetal birth weight) and serum AHSG levels in preeclampsia (data not shown).
Diagnostic accuracy of serum AHSG measurements in preeclampsia
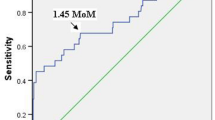
Using the receiver operating characteristic curve analysis, we determined a cutoff value of AHSG concentration (720 μg ml−1) that can discriminate preeclamptic patients from normotensive, healthy pregnant women with 68.1% sensitivity and 60.8% specificity. A low AHSG level (⩽720 μg ml−1) was significantly associated with preeclampsia (odds ratio: 3.32, 95% confidence interval: 1.88–5.86; P<0.001), even after adjustments for maternal age, BMI and gestational age at blood draw in multiple logistic regression analysis (adjusted odds ratio with 95% confidence interval: 3.69 (1.82–7.51); P<0.001).
We compared the diagnostic performance of serum AHSG and CRP measurements in preeclampsia. As displayed in Figure 2, there was no significant difference between the areas under the receiver operating characteristic curves (AUCs) of AHSG and CRP (AUC for AHSG and CRP with 95% confidence interval were 0.68 (0.61–0.74) and 0.65 (0.58–0.72), respectively; P=0.61).
Power analysis
The power of our study to detect the observed differences in serum AHSG levels between the preeclamptic and the control group, at a type I error rate of 0.05, was 99.8%.
Discussion
In this study, we found that serum AHSG concentrations were decreased and showed inverse correlations with systolic blood pressure and serum CRP levels in preeclampsia. The measurement of serum AHSG concentrations was observed to be as accurate as that of serum CRP levels to detect preeclampsia.
Several mechanisms have been implicated in regulating serum AHSG concentrations. The protein is synthesized predominantly in the liver, and its serum level is decreased in patients with liver cirrhosis and hepatocellular cancer because of an impaired hepatic synthetic capacity.25, 26 The synthesis of human AHSG in the liver is downregulated by pro-inflammatory cytokines, indicating that this protein is a negative acute-phase reactant.27 Estrogen might also influence the production of the protein, as suggested by the changes in its serum levels after menopause and estrogen therapy.28 In addition, an impaired renal function was observed to be associated with decreased circulating AHSG levels, and the degree of glomerular dysfunction correlated with AHSG levels in patients with chronic kidney disease.29 Single-nucleotide polymorphisms of the gene encoding AHSG were also shown to affect the serum levels of the protein in different populations.30, 31 Furthermore, an increased consumption was also supposed to result in reduced serum AHSG levels in patients with hematological malignancies.32 In pregnancy, the fetoplacental unit can be another source of the protein. Indeed, AHSG is highly expressed in placental tissues, and a passive transplacental transfer of this substance has also been suggested.33, 34 Our research group has previously reported that serum AHSG concentration increases with advancing gestation in normal pregnancy.35
According to our findings, the maternal systemic inflammatory response, which involves an acute-phase reaction, accounts—at least partly—for the decreased serum AHSG levels in preeclampsia, as suggested by the significant inverse correlation with serum CRP concentrations. In fact, circulating AHSG levels decrease in several conditions characterized by tissue damage, infection, inflammation or malignancy.16, 32, 36, 37, 38 However, other factors may also have contributed to our observation. It has been shown earlier that AHSG enhances the macrophage-mediated phagocytosis of apoptotic cells in vitro by stimulating macropinocytosis.39 Therefore, it is also possible that the reduced serum AHSG concentration in preeclampsia is a consequence of the consumption of the protein during the clearance of trophoblast debris shed into maternal circulation. Renal protein excretion might provide an additional explanation for decreased circulating AHSG levels in preeclampsia.40 Nevertheless, the serum and urine levels of this protein did not correlate in patients with various renal diseases.41 In preeclampsia, decreased estrogen levels and action have been observed in previous studies,10, 42 which might also result in lower AHSG concentrations. Moreover, the role of AHSG gene polymorphisms has not been investigated yet in this pregnancy-specific disorder.
The diagnostic value of the serum AHSG measurement was detected to be similar to that of serum CRP levels in preeclampsia. CRP is a sensitive marker of tissue damage and inflammation. Several studies have indicated that its serum concentration is higher in preeclamptic patients than in healthy pregnant women.43, 44 In addition, raised CRP levels were shown to precede the development of preeclampsia.45 However, obesity, which is by itself an inflammatory condition, confounded these observations.46, 47 Although AHSG can slightly modulate body mass,48 the low serum level of this protein was independently associated with an increased risk of preeclampsia in our study. Nevertheless, the large overlap in serum AHSG and CRP levels between preeclamptic patients and healthy pregnant women—reflected also by sensitivity and specificity values—suggests that mechanisms other than systemic inflammation are also involved in the pathogenesis of this multifactorial disorder.
The serum AHSG levels did not differ significantly between our preeclamptic patients with and without fetal growth restriction. We have found earlier that AHSG along with tumor necrosis factor-α and leptin may negatively regulate neonatal skeletal development.35 In a recent study, no significant differences were observed in maternal, fetal and neonatal AHSG concentrations between pregnancies with normal and asymmetrically restricted fetal growth,34 which is consistent with our findings. Beyond its concentration, the post-translational modifications of the protein (phosphorylation and glycosylation) can also influence the biological activity of AHSG.49, 50 Indeed, prominent defects in the sialylation of the protein were found in the plasma of neonates with intrauterine growth restriction.50
A low circulating AHSG level may not only be a marker for preeclampsia but might also be involved in its pathogenesis. In patients with chronic kidney disease, a low AHSG (fetuin-A) concentration has been implicated in the development of endothelial dysfunction,29, 51 which is also a hallmark of preeclampsia. Furthermore, AHSG, as a mineral chaperone, can prevent extraskeletal (soft tissue) calcification.52 It is noteworthy that increased arterial stiffness, which has been shown to be associated with vascular calcification,53 has been detected in preeclamptic pregnancies.54 Interestingly, systolic blood pressure, which represents the viscoelastic properties of large conduit vessels rather than the vasoconstriction of resistance arteries, was inversely correlated with serum AHSG levels in our preeclamptic group. Several studies revealed the anti-inflammatory role of AHSG. The protein inhibits the production of the pro-inflammatory cytokine, interleukin-2, by peripheral blood lymphocytes.55 In addition, AHSG, as an opsonin, was required for macrophage deactivation in vitro.56 AHSG also attenuated tumor necrosis factor-α synthesis and the innate inflammatory response in an experimental model.57 Moreover, the protein has been shown to enhance the phagocytosis of apoptotic cells by macrophages.39 In pregnancy, a decreased clearance of necrotic and apoptotic trophoblast cells may lead to an increased shedding of placental debris into the maternal circulation, resulting in an excessive maternal systemic inflammatory response characteristic of preeclampsia.
On the other hand, a decreased serum AHSG level can also have beneficial effects in pregnancy. The inadequate production of transforming growth factor-β by leukocytes may cause poor angiogenesis, resulting in preeclampsia.8 AHSG, similar to soluble endoglin, can inhibit transforming growth factor-β activity.58 Insulin resistance is a definite risk factor for preeclampsia.2 According to our previous findings, AHSG may contribute to insulin resistance during normal pregnancy and gestational diabetes.35
In spite of its strength due to the large number of preeclamptic patients and healthy pregnant women involved, our study is limited by its case–control design. A prospective study should be undertaken to determine whether a decrease in the serum AHSG concentration precedes the development of preeclampsia, and thus can help predict this serious complication in pregnancy.
In conclusion, serum AHSG concentration is decreased in preeclampsia, which reflects—at least partly—systemic inflammation. However, further studies are required to shed light on the biological functions of circulating AHSG in normal pregnancy and preeclampsia.
References
Duckitt K, Harrington D . Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005; 330: 565.
Sibai B, Dekker G, Kupferminc M . Pre-eclampsia. Lancet 2005; 365: 785–799.
Redman CW, Sacks GP, Sargent IL . Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999; 180: 499–506.
Redman CW, Sargent IL . Latest advances in understanding preeclampsia. Science 2005; 308: 1592–1594.
Redman CW, Sargent IL . Preeclampsia and the systemic inflammatory response. Semin Nephrol 2004; 24: 565–570.
Saito S, Sakai M . Th1/Th2 balance in preeclampsia. J Reprod Immunol 2003; 59: 161–173.
Sargent IL, Borzychowski AM, Redman CW . Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online 2006; 13: 680–686.
Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y . The role of the immune system in preeclampsia. Mol Aspects Med 2007; 28: 192–209.
Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M . Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res 2007; 30: 151–159.
Molvarec A, Ver A, Fekete A, Rosta K, Derzbach L, Derzsy Z, Karadi I, Rigo J Jr . Association between estrogen receptor alpha (ESR1) gene polymorphisms and severe preeclampsia. Hypertens Res 2007; 30: 205–211.
Molvarec A, Jermendy A, Kovacs M, Prohaszka Z, Rigo J Jr . Toll-like receptor 4 gene polymorphisms and preeclampsia: lack of association in a Caucasian population. Hypertens Res 2008; 31: 859–864.
Canto P, Canto-Cetina T, Juarez-Velazquez R, Rosas-Vargas H, Rangel-Villalobos H, Canizales-Quinteros S, Velazquez-Wong AC, Villarreal-Molina MT, Fernandez G, Coral-Vazquez R . Methylenetetrahydrofolate reductase C677T and glutathione S-transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya-Mestizo women. Hypertens Res 2008; 31: 1015–1019.
Zafarmand MH, Franx A, Sabour S, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML . The M235T variant of the angiotensinogen gene is related to development of self-reported hypertension during pregnancy: the Prospect-EPIC cohort study. Hypertens Res 2008; 31: 1299–1305.
Hirashima C, Ohkuchi A, Matsubara S, Suzuki H, Takahashi K, Usui R, Suzuki M . Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens Res 2008; 31: 1541–1548.
Arnaud P, Kalabay L . Alpha2-HS glycoprotein: a protein in search of a function. Diabetes Metab Res Rev 2002; 18: 311–314.
Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G . Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest 1979; 64: 1118–1129.
Hruska KA, Saab G, Chaudhary LR, Quinn CO, Lund RJ, Surendran K . Kidney–bone, bone–kidney, and cell–cell communications in renal osteodystrophy. Semin Nephrol 2004; 24: 25–38.
Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, Grunberger G . Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 1993; 7: 1445–1455.
Lewis JG, Andre CM . Enhancement of human monocyte phagocytic function by alpha 2HS glycoprotein. Immunology 1981; 42: 481–487.
van Oss CJ, Gillman CF, Bronson PM, Border JR . Opsonic properties of human serum alpha-2 hs glycoprotein. Immunol Commun 1974; 3: 329–335.
Dziegielewska KM, Mollgard K, Reynolds ML, Saunders NR . A fetuin-related glycoprotein (alpha 2HS) in human embryonic and fetal development. Cell Tissue Res 1987; 248: 33–41.
Molvarec A, Prohaszka Z, Nagy B, Kalabay L, Szalay J, Fust G, Karadi I, Rigo J Jr . Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol 2007; 73: 172–179.
National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183: S1–S22.
Joubert K . Standards of the body mass and body length of birth in Hungary on the basis of the 1990–1996 nation-wide liveborn data. Magy Noorv Lapja 2000; 63: 155–163.
Kalabay L, Szalay F, Nemesanszky E, Telegdy L, Jakab L, Romics L . Decreased serum alfa2-HS-glycoprotein concentration in patients with primary biliary cirrhosis. J Hepatol 1997; 26: 1426–1427.
Kalabay L, Jakab L, Prohaszka Z, Fust G, Benko Z, Telegdy L, Lorincz Z, Zavodszky P, Arnaud P, Fekete B . Human fetuin/alpha2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur J Gastroenterol Hepatol 2002; 14: 389–394.
Daveau M, Christian D, Julen N, Hiron M, Arnaud P, Lebreton JP . The synthesis of human alpha-2-HS glycoprotein is down-regulated by cytokines in hepatoma HepG2 cells. FEBS Lett 1988; 241: 191–194.
Hashimoto S, Miwa M, Akasofu K, Nishida E . Changes in 40 serum proteins of post-menopausal women. Maturitas 1991; 13: 23–33.
Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Sonmez A, Eyileten T, Yenicesu M, Oguz Y, Tasar M, Vural A, Ikizler TA, Stenvinkel P, Lindholm B . Serum fetuin—a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract 2008; 108: c233–c240.
Osawa M, Tian W, Horiuchi H, Kaneko M, Umetsu K . Association of alpha2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Hum Genet 2005; 116: 146–151.
Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, Barany P, Lindholm B, Jogestrand T, Heimburger O, Holmes C, Schalling M, Nordfors L . Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney Int 2005; 67: 2383–2392.
Kalabay L, Cseh K, Benedek S, Fekete S, Masszi T, Herjeczki K, Pozsonyi T, Jakab L, Jakab L . Serum alpha 2-HS glycoprotein concentration in patients with hematological malignancies. A follow-up study. Ann Hematol 1991; 63: 264–269.
Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W . Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J 2003; 376: 135–145.
Briana DD, Boutsikou M, Gourgiotis D, Boutsikou T, Baka S, Marmarinos A, Hassiakos D, Malamitsi-Puchner A . Serum fetuin-A/alpha2-HS-glycoprotein in human pregnancies with normal and restricted fetal growth. J Matern Fetal Neonatal Med 2008; 21: 826–830.
Kalabay L, Cseh K, Pajor A, Baranyi E, Csakany GM, Melczer Z, Speer G, Kovacs M, Siller G, Karadi I, Winkler G . Correlation of maternal serum fetuin/alpha2-HS-glycoprotein concentration with maternal insulin resistance and anthropometric parameters of neonates in normal pregnancy and gestational diabetes. Eur J Endocrinol 2002; 147: 243–248.
Kalabay L, Jakab L, Cseh K, Pozsonyi T, Jakab LA . Correlations between serum alpha 2-HS-glycoprotein concentration and conventional laboratory parameters in systemic lupus erythematosus. Acta Med Hung 1990; 47: 53–64.
Kalabay L, Cseh K, Jakab L, Pozsonyi T, Jakab L, Benedek S, Fekete S, Telegdy L . Diagnostic value of the determination of serum alpha2-HS-glycoprotein. Orv Hetil 1992; 133: 1553–1554; 1559–1560.
Mathews ST, Deutsch DD, Iyer G, Hora N, Pati B, Marsh J, Grunberger G . Plasma alpha2-HS glycoprotein concentrations in patients with acute myocardial infarction quantified by a modified ELISA. Clin Chim Acta 2002; 319: 27–34.
Jersmann HP, Dransfield I, Hart SP . Fetuin/alpha2-HS glycoprotein enhances phagocytosis of apoptotic cells and macropinocytosis by human macrophages. Clin Sci (Lond) 2003; 105: 273–278.
Shinagawa S, Saitoh M . A study on proteins contained in urine of gestosis patients. Biol Res Pregnancy Perinatol 1983; 4: 140–144.
Kishore BK, Gejyo F, Arakawa M . Alpha 2HS-glycoprotein in the serum and urine of patients with renal diseases. Postgrad Med J 1983; 59: 304–307.
Rahman SA, Hingorani V, Laumas KR . Biosynthesis of oestrogens and their inter-conversion in human placentae from normal and toxaemic pregnancies. Clin Endocrinol (Oxf) 1975; 4: 333–341.
Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P . Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet 2001; 75: 243–249.
Ustun Y, Engin-Ustun Y, Kamaci M . Association of fibrinogen and C-reactive protein with severity of preeclampsia. Eur J Obstet Gynecol Reprod Biol 2005; 121: 154–158.
Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ . Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol 2003; 59: 29–37.
Belo L, Santos-Silva A, Caslake M, Cooney J, Pereira-Leite L, Quintanilha A, Rebelo I . Neutrophil activation and C-reactive protein concentration in preeclampsia. Hypertens Pregnancy 2003; 22: 129–141.
Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA . A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens 2004; 17: 154–160.
Lavebratt C, Wahlqvist S, Nordfors L, Hoffstedt J, Arner P . AHSG gene variant is associated with leanness among Swedish men. Hum Genet 2005; 117: 54–60.
Haglund AC, Ek B, Ek P . Phosphorylation of human plasma alpha2-Heremans–Schmid glycoprotein (human fetuin) in vivo. Biochem J 2001; 357: 437–445.
Karamessinis PM, Malamitsi-Puchner A, Boutsikou T, Makridakis M, Vougas K, Fountoulakis M, Vlahou A, Chrousos G . Marked defects in the expression and glycosylation of alpha2-HS glycoprotein/fetuin-A in plasma from neonates with intrauterine growth restriction: proteomics screening and potential clinical implications. Mol Cell Proteomics 2008; 7: 591–599.
Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Eyileten T, Sonmez A, Oguz Y, Oner K, Ors F, Vural A, Yenicesu M . Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation 2007; 83: 392–397.
Jahnen-Dechent W, Schafer C, Ketteler M, McKee MD . Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med 2008; 86: 379–389.
Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM . Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001; 38: 938–942.
Tihtonen KM, Koobi T, Uotila JT . Arterial stiffness in preeclamptic and chronic hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol 2006; 128: 180–186.
Jakab L, Jakab L, Kalabay L, Pozsonyi T, Cseh K . The effect of the alpha 2-HS-glycoprotein on the mitogen-induced lymphoblastic transformation and IL-2 production. Acta Physiol Hung 1991; 77: 25–31.
Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ . Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci USA 1998; 95: 14429–14434.
Ombrellino M, Wang H, Yang H, Zhang M, Vishnubhakat J, Frazier A, Scher LA, Friedman SG, Tracey KJ . Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock 2001; 15: 181–185.
Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW . Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem 1996; 271: 12755–12761.
Acknowledgements
The skilful technical assistance of Szigeti Antalné and Nagyné Vers Mária and the support of Szilvia Walentin, Éva Imreh and Mónika Kleiber (Central Laboratory, Kútvölgyi Clinical Center, Semmelweis University, Budapest, Hungary) are gratefully acknowledged. This work was supported by research grants from the Hungarian Scientific Research Fund (NF 72689) and the Faculty of Medicine of the Semmelweis University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molvarec, A., Kalabay, L., Derzsy, Z. et al. Preeclampsia is associated with decreased serum α2-HS glycoprotein (fetuin-A) concentration. Hypertens Res 32, 665–669 (2009). https://doi.org/10.1038/hr.2009.79
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/hr.2009.79
Keywords
This article is cited by
-
Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array
BMC Immunology (2010)
-
G protein-coupled receptor kinase 4 gene variants are not associated with preeclampsia in Northern Han Chinese
Hypertension Research (2010)
-
Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia
Hypertension Research (2010)
-
Is increased maternal endotelin-1 concentration associated with neonatal asphyxia and preterm delivery in intrahepatic cholestasis of pregnancy?
Archives of Gynecology and Obstetrics (2010)