Abstract
Objective:
To investigate the anti-obesity effect of Rubi Fructus (RF) extract using brown adipose tissue (BAT) and primary brown preadipocytes in vivo and in vitro.
Methods:
Male C57BL/6 J mice (n=5 per group) were fed a high-fat diet (HFD) for 10 weeks with or without RF. Brown preadipocytes from the interscapular BAT of mice (age, post-natal days 1–3) were cultured with differentiation media (DM) including isobutylmethylxanthine, dexamethasone, T3, indomethacin and insulin with or without RF.
Results:
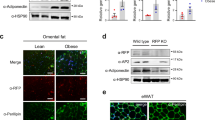
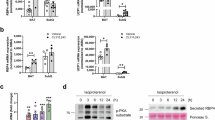
In HFD-induced obese C57BL/6 J mice, long-term RF treatment significantly reduced weight gain as well as the weights of the white adipose tissue, liver and spleen. Serum levels of total cholesterol and low-density lipoprotein cholesterol were also reduced in the HFD group which received RF treatment. Furthermore, RF induced thermogenic-, adipogenic- and mitochondria-related gene expressions in BAT. In primary brown adipocytes, RF effectively stimulated the expressions of thermogenic- and mitochondria-related genes. In addition, to examine whether LIPIN1, a regulator of adipocyte differentiation, is regulated by RF, Lipin1 small interfering RNA (siRNA) and RF were pretreated in primary brown adipocytes. Pretreatment with Lipin1 siRNA and RF downregulated the DM-induced expression levels of thermogenic- and mitochondria-related genes. Moreover, RF markedly upregulated AMP-activated protein kinase. Our study shows that RF is capable of stimulating the differentiation of brown adipocytes through the modulation of thermogenic genes.
Conclusions:
This study demonstrates that RF prevents the development of obesity in mice fed with a HFD and that it is also capable of stimulating the differentiation of brown adipocytes through the modulation of thermogenic genes, which suggests that RF has potential as a therapeutic application for the treatment or prevention of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stumvoll M, Goldstein BJ, van Haeften TW . Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333–1346.
Kim KS, Yang HJ, Choi EK, Shin MH, Kim KH, Um JY et al. The effects of complex herbal medicine composed of Cornus fructus, Dioscoreae rhizoma, Aurantii fructus, and Mori folium in obese type-2 diabetes mice model. Orient Pharm Exp Med 2013; 13: 69–75.
Kopelman PG . Obesity as a medical problem. Nature 2000; 404: 635–643.
Collins S, Cao W, Daniel KW, Dixon TM, Medvedev AV, Onuma H et al. Adrenoceptors, uncoupling proteins, and energy expenditure. Exp Biol Med (Maywood) 2001; 226: 982–990.
Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B . UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 2001; 1504: 82–106.
Sell H, Deshaies Y, Richard D . The brown adipocyte: update on its metabolic role. Int J Biochem Cell Biol 2004; 36: 2098–2104.
Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ . BAT: a new target for human obesity? Trends Pharmacol Sci 2009; 30: 387–396.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23: 3113–3120.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Ceddia RB . The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol 2013; 366: 194–203.
Peterfy M, Phan J, Xu P, Reue K . Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nature Genet 2001; 27: 121–124.
Peterfy M, Phan J, Reue K . Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J Biol Chem 2005; 280: 32883–32889.
van Harmelen V, Ryden M, Sjolin E, Hoffstedt J . A role of lipin in human obesity and insulin resistance: relation to adipocyte glucose transport and GLUT4 expression. J Lipid Res 2007; 48: 201–206.
Croce MA, Eagon JC, LaRiviere LL, Korenblat KM, Klein S, Finck BN . Hepatic lipin 1 beta expression is diminished in insulin-resistant obese subjects and is reactivated by marked weight loss. Diabetes 2007; 56: 2395–2399.
Phan J, Reue K . Lipin a lipodystrophy and obesity gene. Cell Metab 2005; 1: 73–83.
Lim JW, Jeong JT, Shin CS . Component analysis and sensory evaluation of Korean black raspberry (Rubus coreanus Mique) wines. Int J Food Sci Tech 2012; 47: 918–926.
Lim JW, Hwang HJ, Shin CS . Polyphenol compounds and anti-inflammatory activities of Korean black raspberry ( Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J Agric Food Chem 2012; 60: 5121–5127.
Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ et al. Antinociceptive and antiinflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull 2003; 26: 1436–1441.
Lee JE, Park E, Auh JH, Choi HK, Lee J, Cho S et al. Effects of a Rubus coreanus Miquel supplement on plasma antioxidant capacity in healthy Korean men. Nutr Res Pract 2011; 5: 429–434.
Jeong MY, Kim HL, Park J, An HJ, Kim SH, Kim SJ et al. Rubi fructus (rubus coreanus) inhibits differentiation to adipocytesc in 3T3-L1 cells. Evid Based Complement Alternat Med 2013; 2013: 475386.
Liu Y, Dang HX, Li D, Pang W, Hammock BD, Zhu Y . Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One 2012; 7: e39165.
Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR . beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem 1999; 274: 34795–34802.
Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR . Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol 2004; 24: 1918–1929.
Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W . Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992; 97: 493–497.
Dugani CB, Klip A . Glucose transporter 4: cycling, compartments and controversies. EMBO Rep 2005; 6: 1137–1142.
Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009; 460: 1154–1158.
Kraus D, Fasshauer M, Ott V, Meier B, Jost M, Klein HH et al. Leptin secretion and negative autocrine crosstalk with insulin in brown adipocytes. J Endocrinol 2002; 175: 185–191.
Viengchareun S, Zennaro MC, Pascual-Le Tallec L, Lombes M . Brown adipocytes are novel sites of expression and regulation of adiponectin and resistin. FEBS Lett 2002; 532: 345–350.
Higashida K, Higuchi M, Terada S . Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem Biophys Res Commun 2008; 374: 587–591.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004.
Yang X, Enerback S, Smith U . Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obesity Res 2003; 11: 1182–1191.
Scarpulla RC . Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 2002; 286: 81–89.
Shi T, Wang F, Stieren E, Tong Q . SIRT3 a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 2005; 280: 13560–13567.
Glatz JF, Borchers T, Spener F, van der Vusse GJ . Fatty acids in cell signalling: modulation by lipid binding proteins. Prostaglandins Leukot Essent Fatty Acids 1995; 52: 121–127.
Kadowaki T, Yamauchi T . Adiponectin and adiponectin receptors. Endocrine Rev 2005; 26: 439–451.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM et al. The hormone resistin links obesity to diabetes. Nature 2001; 409: 307–312.
Rajala MW, Obici S, Scherer PE, Rossetti L . Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest 2003; 111: 225–230.
Suviolahti E, Reue K, Cantor RM, Phan J, Gentile M, Naukkarinen J et al. Cross-species analyses implicate Lipin 1 involvement in human glucose metabolism. Hum Mol Genet 2006; 15: 377–386.
Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN et al. Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor gamma activation. Diabetes 2006; 55: 2811–2818.
Nadra K, Medard JJ, Mul JD, Han GS, Gres S, Pende M et al. Cell autonomous lfunction is essential for development and maintenance of white and brown adipose tiipin 1 function is essential for development and maintenance of white and brown adipose tissue. Mol Cell Biol 2012; 32: 4794–4810.
Vila-Bedmar R, Lorenzo M, Fernandez-Veledo S . Adenosine 5'-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 2010; 151: 980–992.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (no. 2011-0030130, no. 2011–0006220, no. 2012M2B2B1055244, no. 2013R1A2A2A03006068, no. 2010-0022450 and 2013R1A1A2059601).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Jeong, MY., Kim, HL., Park, J. et al. Rubi Fructus (Rubus coreanus) activates the expression of thermogenic genes in vivo and in vitro. Int J Obes 39, 456–464 (2015). https://doi.org/10.1038/ijo.2014.155
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2014.155