Abstract
Objectives:
Cytochrome oxidase (COX) dysfunction is associated with mitochondrial oxidative stress. We determined the association between COX expression, obesity and type 2 diabetes.
Subjects/methods:
COX4I1 and COX10 genes were measured in monocytes of 24 lean controls, 31 glucose-tolerant and 67 diabetic obese patients, and 17 morbidly obese patients before and after bariatric surgery. We investigated the effect of caloric restriction and peroxisome proliferator-activated receptor (PPAR) agonist treatment on Cox in obese diabetic mice, and that of diet-induced insulin resistance in Streptozotocin-treated mice.
Results:
Low COX4I1 was associated with type 2 diabetes in obese patients, adjusting for age, gender, smoking, interleukin-6 and high-sensitivity C-reactive protein, all related to metabolic syndrome (MetS; odds ratio: 6.1, 95% confidence interval: 2.3–16). In contrast, COX10 was low in glucose-tolerant and diabetic obese patients. In morbidly obese patients, COX4I1 was lower in visceral adipose tissue collected at bariatric surgery. In their monocytes, COX4I1 decreased after bariatric surgery, and low COX4I1 at 4 months was associated with MetS at 7 years. In leptin-deficient obese diabetic mice, Cox4i1 was low in white visceral adipose tissue (n=13; P<0.001) compared with age-matched lean mice (n=10). PPARγ-agonist treatment (n=13), but not caloric restriction (n=11), increased Cox4i1 (P<0.001). Increase in Cox4i1 depended on the increase in glucose transporter 4 ( Glut4) expression and insulin sensitivity, independent of the increase in blood adiponectin. In streptozotocin-treated mice (three groups of seven mice, diet-induced insulin resistance decreased Cox4i1 and Glut4 (P<0.001 for both).
Conclusion:
COX4I1 depression is related to insulin resistance and type 2 diabetes in obesity. In peripheral blood monocytes, it may be a diagnostically useful biomarker.
Similar content being viewed by others
Introduction
Obesity is an important risk factor for metabolic syndrome (MetS), type 2 diabetes and cardiovascular disease.1, 2, 3 Low-grade inflammation has been shown to underlie the development of obesity-related complications.4, 5, 6 Recent studies have supported the contention that increased oxidative stress in adipose tissue is an early instigator of MetS.7, 8, 9, 10, 11 Oxidative damage of adipose tissues is associated with impaired adipocyte maturation and production of pro-inflammatory adipocytokines by dysfunctional adipocytes, emphasizing an important role of adiponectin.12, 13 Both factors increase infiltration of activated monocytes into the adipose tissues where they differentiate into macrophages that produce inflammatory chemokines.14 The existence of specific markers for type 2 diabetes in relation to oxidative stress in adipose tissues is yet to be determined. Moreover, if these markers would be available with a simple peripheral blood test, they would be a useful clinical tool to predict the risk of type 2 diabetes.
Obesity, MetS, type 2 diabetes and cardiovascular diseases were found to be associated with systemic oxidative stress, mainly evidenced by high levels of oxidized low-density lipoprotein (LDL).8, 9, 10, 15 It has been proposed that mitochondrial decline resulting in mitochondrial oxidative stress contributes to the development of age-related metabolic and cardiovascular diseases.16 However, the relation of mitochondrial oxidative stress and those diseases remains to be elucidated. Dysfunction of the cytochrome oxidase (COX) complex, the terminal node and rate-limiting step in the mitochondrial electron transport chain, is associated with mitochondrial oxidative stress,17, 18 a condition associated with obesity, MetS and type 2 diabetes.9, 10, 19, 20 COX4I1 is suggested to be the most important regulatory subunit of COX21, 22 as it is required for the allosteric feedback inhibition of the enzyme by its indirect product ATP. COX10 as this subunit is required for COX biogenesis.23 Hence, the aims of this study were to investigate the association of mitochondrial oxidative stress with obesity, MetS and type 2 diabetes24 and to test the potential of COX4I1 and COX10 in peripheral blood monocytes as biomarkers for a detrimental metabolic evolution in patients with obesity. To this end, we determined their expressions in adipose tissue and in monocytes in representative animal models and patient populations.
Subjects and methods
Subjects
The first patient group consisted of 98 successive obese individuals: 31 normal glucose-tolerant obese (NGTO) patients and 67 obese patients with type 2 diabetes, according to the American Diabetes Association. Inclusion was blinded. The samples were collected at the Division of Endocrinology between 27 September and 20 December 2007. Obese subjects were compared with 24 lean control persons. All participants were without symptoms of clinical atherosclerotic cardiovascular disease. This study complies with the Declaration of Helsinki and the Medical Ethics Committee of the KU Leuven approved the study protocol. All human participants gave written informed consent.
The second patient cohort comprised 17 obese individuals. These 17 morbidly obese subjects were referred to our hospital for bariatric surgery. After multidisciplinary deliberation, the selected patients received a laparoscopic Roux-en-Y gastric bypass. A 30-ml fully divided gastric pouch was created and the jejunum, 30 cm distal of the ligament of Treitz, was anastomosed to it with a circular stapler of 25 mm. To restore intestinal transit, a fully stapled entero–entero anastomose was constructed 120 cm distal on the alimentary limb. In this way, the food passage was derived away from almost the whole stomach, the duodenum and the proximal jejunum.25, 26, 27 Monocytes were isolated from venous blood taken before, and at 4 months and 7 years after bariatric surgery. Visceral adipose tissue biopsies were collected at the time of surgery. The samples were collected between 29 March 2005 and 28 February 2013.
Plasma analysis
Blood samples were centrifuged to prepare plasma samples. Total, high-density lipoprotein (HDL) cholesterol, triglycerides, adiponectin, oxidized LDL (Ox-LDL), high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6) were measured as before.28 LDL cholesterol levels were calculated using the Friedewald formula. Plasma glucose was measured using the glucose oxidase method (Johnson & Johnson, Zaventem, Belgium), and insulin with an immunoassay (Biosource Technologies, Fleunes, Belgium).29, 30 Insulin resistance was calculated by a homeostasis model assessment of insulin resistance (HOMA-IR)=fasting plasma insulin (mU l−1) x fasting blood glucose (mM)/22.5.31 All laboratory assessments were performed without the knowledge of clinical data. Blood pressure was taken three times with the participant in a seated position after 5 min of quiet rest, and the average of the last two measurements was used. MetS was defined according to the joint interim statement of 2009.1
Monocyte isolation from human blood
Peripheral blood mononuclear cells (PBMCs) were prepared from the anticoagulated blood using gradient separation on Histopaque-1077. Monocytes were isolated from PBMCs (1 × 107 cells) using anti-CD14 coated beads, and an LS column that was put in a MidiMACS Separator (Miltenyi Biotec Ltd., Surrey, UK).32, 33
Mouse studies
Animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the KU Leuven (P087/2007). Breeding and genotyping of mice deficient in LDL receptor (Jackson Laboratory, Bar Harbor, ME, USA) and leptin ob gene (Jackson Laboratory; DKO), backcrossed on the C57BL/6J background (KU Leuven), were performed as previously described.29, 30 Food intake of male DKO mice was ≈5.7 g per day; this is almost double of the mean food intake of lean mice (≈3 g per day). We studied placebo DKO (n=13), DKO mice in which the weight loss was induced by caloric restriction ((≈3 g per day; n=11) and DKO mice treated with rosiglitazone (n=13). Randomization was blinded. Mice were included in the study at 12 weeks and were followed during 12 weeks. They were compared with age- and gender-matched lean C57BL/6J mice (n=10). Rosiglitazone (Sigma Aldrich, Diegem, Belgium, 10 mg kg−1 per day) was added to standard chow containing 4% fat (Ssniff, Soest, Germany), placebo-treated mice received the grinded chow only. Rosiglitazone did not affect the food intake. Food and water were available ad libitum. At baseline (12 weeks), characteristics of group DKO mice were identical.
Because DKO mice are leptin deficient, we also wanted to study mice with impaired insulin signaling that overexpress leptin as in obese persons. In brief, male C57BL/6 mice received a single subcutaneous injection of Streptozotocin (STZ; Sigma Aldrich) 2 days after birth (performed at Stelic (Tokyo, Japan) under the supervision of Paul Holvoet. In this experiment, mice were fed a high-fat diet (CLEA, Tokyo, Japan) ad libitum after 4 weeks of age. The composition was as follows: 24.5% casein, 5% egg white powder, 0.4% l-Cystine, 15.9% beef tallow powder (80%), 20% safflower (high oleic acid), 5.5% cellulose; 8.3% maltodextrin and 6.9% lactose; 6.8% sucrose.34, 35 Mice were studied at 4, 8 and 12 weeks after STZ injection (seven mice per group) and were compared with age-matched C57BL/6 mice (six, six and nine mice per group). All mice were killed by Nembutal overdose.29, 30 No animals were excluded.
The investigator was blinded to the group allocation during the experiment and when assessing the outcome.
RNA isolation and quantitative real-time PCR analysis
Extraction and purification of RNA and complementary DNA generation was performed as described before.29, 36, 37 qPCR was performed on a 7500 Fast Real-Time PCR system using Fast SYBRGreen master mix (Applied Biosystems, Ghent, Belgium). Oligonucleotides (Invitrogen, Ghent, Belgium) used as forward and reverse primers were designed using the ‘Primer Express’ software (Applied Biosystems; Supplementary Table 1). RNA expression levels were calculated with the delta-delta-quantification cycle method (ΔΔCq).38 β-actin for mouse experiments, and HPRT1, SDHA, TBP and YWHAZ for patient samples were selected as most stable housekeeping genes using GeNorm.39
In order to test the reproducibility and variance of expression of RNA markers, RNA expression was measured in monocytes of 13 healthy individuals collected at weeks 0, 1 and 2. P-values, determined by repeated measures test, were 0.95 for COX1, 0.96 for COX4I1, 0.92 for glutathione peroxidase (GPX1) and 0.88 for IRAK3. Receiver operating characteristic (ROC) curves of week 0 and week 1 and week 2 were also compared. Mean areas under the curves (AUC) were 0.56 for COX10, 0.54 for COX4I1, 0.53 for GPX1 and 0.54 for IRAK3, whereby an ROC of around 0.5 indicates no evidence that the data are different according to time of collection. Mean within-group variability was 14% for COX10, 12% for COX4I1 and 17% for GPX1 and IRAK3.
Statistics
Two groups were compared using an unpaired Mann–Whitney U-test. More than two groups were compared using nonparametric one-way analysis of variance (Kruskal–Wallis) followed by comparison by the Dunn’s multiple comparison test or by repeated measures analysis of variance followed by the Bonferroni’s multiple comparison test. It was tested whether variance in the groups that were being statistically compared was similar. Odds ratios were determined by Fisher exact test. All analyses were performed using GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego, CA, USA), except for ROC analysis, where MedCalc statistical software (Medcalc Software bvba, Ostend, Belgium) for biomedical research was used. A P-value <0.05 was considered statistically significant. We used the on-line Power & Sample Size Calculator of Statistical Solutions to determine if the sample size for each study assured an adequate power (at least 0.80) to detect statistical significance (at least P<0.05).
Results
Relation of RNA expressions to type 2 diabetes in patients with obesity
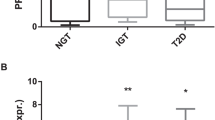
We determined the relation between RNA expressions and diabetes in a cohort of 31 NGTO patients and 67 obese patients with type 2 diabetes. Compared with 24 lean controls, age-matched obese persons with type 2 diabetes more often had MetS. Characteristics for all three populations are given in Table 1. NGTOs had higher HOMA-IR, higher diastolic blood pressure and were more often treated with diuretics. Furthermore, they had higher blood levels of leptin, insulin, IL-6, hs-CRP and ox-LDL, but lower levels of adiponectin and HDL cholesterol. Compared with lean controls, obese patients with type 2 diabetes were older, and more often had MetS and used more medication. They had higher systolic blood pressure and diastolic blood pressure, higher triglycerides, leptin, IL-6, hs-CRP and more elevated glycemia and higher HOMA-IR values. Levels of adiponectin and HDL cholesterol were lower. LDL cholesterol was also lower, most likely owing to more frequent statin use. When compared with obese patients without type 2 diabetes, obese patients with diabetes were older, and more often had MetS, higher HOMA-IR and elevated systolic blood pressure. Adiponectin levels were not different, whereas leptin levels were lower. Patients with diabetes also had lower ox-LDL, most probably due to more frequent use of statins. Compared with lean controls, COX10, GPX1 and IRAK3 were low in monocytes of obese patients without and with type 2 diabetes; superoxide dismutase (SOD2) was high. Importantly, COX10 was lower in obese patients with diabetes compared with lean controls and NGTO (Table 1). ROC curve analysis confirmed that COX4I1 was related to type 2 diabetes; its area under the curve (AUC) was higher than 0.70 and its sensitivity was above 70%, similar to that of glucose. More importantly, this test showed an additive value of COX4I1 and glucose (odds ratio increased from 6.6 and 9.5 to 45). The sensitivity and specificity of the combination of COX4I1 and glucose was above 80%. In addition, multiple logistic regression analysis including all genes that were differentially expressed in monocytes of obese persons showed that COX4I1 predicted type 2 diabetes, while adjusting for age, gender, smoking, IL-6 and hs-CRP, which were all related to MetS (odds ratio: 6.1, 95% confidence interval: 2.3–16). In addition, IRAK3 was associated with diabetes (adjusted odds ratio: 4.7, 95% confidence interval: 1.3–17). Together COX4I1 and IRAK3 predicted allocation of 83% of controls without type 2 diabetes and 89% of cases with type 2 diabetes correctly (overall prediction 87%).
Expressions in adipose tissue and monocytes of obese persons and response to bariatric surgery
We compared RNA expressions in visceral adipose tissue of an independent group of 17 obese subjects scheduled for bariatric surgery. Characteristics are shown in Table 2. As expected, obese patients before surgery more often had type 2 diabetes and MetS, and had higher leptin, glucose, HOMA-IR, diastolic blood pressure, IL-6, hs-CRP and Ox-LDL. They had lower blood adiponectin. They were more often treated with oral antidiabetic drugs: seven patients used metformin, two patients used sulfonylurea, whereas no patients were on peroxisome proliferator-activated receptor (PPAR) agonists. COX4I1 (0.79±0.13 vs 1.18±0.30; P<0.05), COX10 (0.88±0.16 vs 1.12±0.23; P<0.05, GPX1 (0.76±0.15 vs 1.21±0.23; P<0.01) and SOD3 (0.66±0.051 vs 0.93±0.094; P<0.05) expressions were significantly lower in the visceral adipose tissue of obese patients when compared with that of the lean controls (n=6). Lower values were associated with impaired adipose tissue differentiation evidenced by lower expressions of ADIPOQ (0.68±0.21 vs 1.43±0.50; P<0.05), GLUT4 (0.37±0.19 vs 1.32±0.53; P<0.01), PPARα (0.65±0.12 vs 1.09±0.37; P<0.01) and PPARβ/δ (0.83±0.12 vs 0.99±0.10; P<0.01), and a trend of lower PPARγ (0.79±0.21 vs 1.13±0.56; P=0.06).
We measured the expressions in monocytes of these 17 patients with obesity. Before bariatric surgery, we found low IRAK3 and high SOD2, as before.28 We also found a decrease of GPX1 in patients with obesity (Table 2), whereas COX4I1 and COX10 were not different when compared with lean controls. Four months after surgery, 16 out of 17 patients still had a body mass index >30 kg m−2, although significant weight loss had occurred and a decreased trend in the number of patients with MetS (from 12 to 6) and type 2 diabetes (from 9 to 5) was observed. Mean levels of leptin, glucose, insulin, triglycerides and systolic blood pressure and diastolic blood pressure were lower, whereas the level of adiponectin was higher. There were no significant changes in drug treatment. At 7 years, 13 out of 17 patients were still obese, despite the persistence of significantly lower body weight. The prevalence of type 2 diabetes remained unchanged when compared with that of patients at 4 months post surgery although HbA1c tended to decrease from 7.1 to 5.9% (54.1 to 41.0 mmol mol−1), not reaching statistical significance. In contrast, there was an increased trend in the number of patients with MetS (from 6 to 9) 7 years after the surgery. Mean levels of leptin, glucose, insulin, triglycerides and ox-LDL, and HOMA-IR and HOMA %b were lower, whereas adiponectin and HDL cholesterol was higher than before surgery. At 7 years, adiponectin and HDL cholesterol were higher and ox-LDL was lower than at 4 months. Interestingly, the levels of glucose, insulin, adiponectin, triglycerides, hs-CRP, ox-LDL and HOMA-IR were similar to those in lean controls, even though body mass index and leptin were still higher than in lean controls. The use of antihypertensive and oral antidiabetic drugs was not statistically different (Table 2).
At 4 months, GPX1 and IRAK3 were still lower and SOD2 was still higher than in lean controls. At 7 years, IRAK3 increased to a level that was even higher than that in lean controls. This was associated with an increase in GPX1 and a decrease in SOD2 that were both similar to the levels observed in lean controls. COX10 was lower at 4 months, and COX10 and COX4I1 were lower at 7 years compared with lean controls and compared with the expression levels 4 months after surgery (Table 2). COX4i1 (1.02±0.24 vs 1.26±0.15; P<0.05) and GPX1 (0.40±0.34 vs 0.76±0.34; P<0.05) was lower at 4 months in patients who had MetS at 7 years compared with those without. Other expressions of investigated markers were not different. Gene expressions before surgery and at 4 months and 7 years after surgery were not different between patients with or without type 2 diabetes. In obese patients who had diabetes, however, COX10 decreased between 4 months and 7 years (−0.12±0.18), whereas in patients who did not have type 2 diabetes COX10 increased (0.17±0.16; P=0.01 for difference).
Mouse studies
The selection of two mouse models allowed us to study the expressions of Cox genes in relation to impaired adipogenesis, hyperglycemia and insulin resistance in leptin-deficient and leptin-sufficient mice.
Obese DKO mice with diabetes had higher weight, hyperglycemia and insulin resistance (evidenced by higher HOMA-IR) and lower plasma levels of adiponectin compared with lean C57BL/6J control mice. Triglyceride and cholesterol levels were higher (Figure 1). RNA expressions of Cox4i1 and Cox10 were lower in white visceral adipose tissues of DKO compared with those in lean mice (Figure 2). Impaired adipogenesis was evidenced by lower expression of glucose transporter 4 (Glut4) and Ppar-α (Figure 2). Pparβ/δ (0.56±0.15 vs 0.99±0.24) and Pparγ (0.22±0.07 vs 0.91±0.17) were also lower (P<0.001 for both). In addition, RNA expressions of Gpx1 and Sod3 were lower (Figure 2).
Characteristics of lean C57BL/6J and obese DKO mice. Scatter dot plots (with mean) of weight, glucose, insulin resistance (HOMA-IR) and blood levels of adiponectin, triglycerides and total cholesterol in lean C57BL/6J control mice (white circles, n=10), placebo DKO (black diamonds, n=13), diet-restricted DKO mice (black squares, n=11), and DKO mice treated with rosiglitazone (black downward triangles, n=13). **P<0.01 and ***P<0.001 compared with lean C57BL/6J mice; †P<0.05, ††P<0.01 and †††P<0.001 compared with placebo DKO mice.
Gene expressions in white visceral adipose tissues of lean and obese mice. Scatter dot plots (with mean) of RNA expressions of Cox4i1, Cox10, Glut4, Pparα, Gpx1 and Sod3 in adipose tissue samples of lean C57BL/6J control mice (white circles, n=10), placebo DKO (black diamonds, n=13), diet-restricted DKO mice (black squares, n=11) and DKO mice treated with rosiglitazone (black downward triangles, n=13). *P<0.05 and ***P<0.001 compared with lean C57BL/6J mice; †P<0.05 and †††P<0.001 compared with placebo DKO mice.
Both caloric restriction and rosiglitazone treatment reduced weight in DKO mice, but the weight of rosiglitazone-treated mice was still higher than that of lean controls. Caloric restriction did not significantly lower glucose and HOMA-IR, but lowered adiponectin. Rosiglitazone treatment reduced glucose, HOMA-IR and adiponectin. None of the interventions lowered triglycerides or cholesterol (Figure 1). Caloric restriction did not increase Cox4i1 and Cox10. In contrast, rosiglitazone treatment increased both (Figure 2). Caloric restriction and rosiglitazone treatment increased Glut4 and PPARα, with a higher increase with rosiglitazone than caloric restriction (P<0.05; Figure 2). In addition, caloric restriction and rosiglitazone treatment increased Pparβ/δ, (0.95±0.19 and 0.75±0.13; P<0.001 for both) and Pparγ (0.43±0.07 and 0.69±0.13; P<0.01 and P<0.001). Both treatments also increased Gpx1 and Sod3 (Figure 2). Cox4i1 was strongly associated with Glut4 (R2=0.48, P<0.001; slope: 0.96±0.15).
Weight of STZ (streptozotocin and high-fat diet-fed) mice was lower, compared with that of control mice, at 4 weeks and at 8 weeks, but not at 12 weeks. STZ mice had hyperglycemia from 4 weeks on and insulin resistance from 8 weeks on (Figure 3). Plasma adiponectin levels of STZ mice were higher at 4 weeks compared with those of control mice. They were not different at 8 weeks, but lower at 12 weeks. They were lower in STZ mice at 8 and 12 weeks than at 4 weeks (Figure 3). Triglyceride levels were higher in STZ mice at 12 weeks, compared with those of control and STZ mice at 4 weeks. Total cholesterol levels were higher in STZ mice at 4, 8 and 12 weeks, compared with those of control and STZ mice at 4 weeks (Figure 3). Plasma leptin levels in control and STZ-treated mice were similar at 4 weeks (1.6±0.6 vs 1.7±1.1 ng ml−1), but were higher in STZ mice at 8 weeks (4.2±1.6 vs 1.4±0.5 ng ml−1) and at 12 weeks (6.6±5.2 ng ml−1 vs 1.3±0.7 ng ml−1; P<0.01). There were no age-dependent changes in weight, glucose, HOMA-IR, adiponectin, triglycerides and total cholesterol in control mice.
Characteristics of lean C57BL/6J and STZ mice. Scatter dot plots (with mean) of weight, glucose, HOMA-IR and plasma levels of adiponectin, triglycerides and total cholesterol in lean control mice at 4 weeks (white circles, n=6), 8 weeks (black circles, n=6) and 12 weeks (white squares, n=9) and STZ mice at 4 weeks (black squares, n=7), 8 weeks (white upward triangles, n=7) and 12 weeks (black upward triangles, n=7). **P<0.01 and ***P<0.001 compared with lean control C57BL/6 J mice; †P<0.05, ††P<0.01 and †††P<0.001 compared with STZ mice at 4 weeks.
Cox4i1 was lower in STZ mice with diabetes at 8 and 12 weeks compared with controls; Cox10 was lower at 12 weeks (Figure 4). Glut4 was lower at 8 and 12 weeks; Pparα was lower at 12 weeks. At all time points, Gpx1 and Sod3 were similar in STZ and in control mice (Figure 4). Pparβ/δ (0.47±0.27 vs 1.02±0.21; P<0.001) was lower at 12 weeks; Pparγ was not different between STZ mice and control. Again, there were no age-dependent changes in RNA expressions in adipose tissues of control mice. Cox4i1 was strongly associated with Glut4 (R2=0.62, P<0.001; slope: 0.85±0.18).
Gene expressions in white visceral adipose tissues of lean C57BL/6J and STZ mice. Scatter dot plots (with mean) of RNA expressions of Cox4i1, Cox10, Glut4, Pparα, Gpx1 and Sod3 in white visceral adipose tissues in control mice at 4 weeks (white circles, n=6), 8 weeks (black circles, n=6) and 12 weeks (white squares, n=9) and STZ mice at 4 weeks (black squares, n=7), 8 weeks (white upward triangles, n=7) and 12 weeks (black upward triangles, n=7). *P<0.05, **P<0.01 and ***P<0.001 compared with age-matched lean control C57BL/6J mice; †P<0.05 and ††P<0.01 compared with STZ mice at 4 weeks.
Discussion
We set out to investigate the association between obesity and type 2 diabetes and low COX4I1 RNA expression. In humans, COX4I1 was lower in monocytes of obese patients with type 2 diabetes compared with NGTO and to lean controls. In both leptin-deficient and leptin-sufficient mice with diabetes, Cox4i1 expression in visceral adipose tissues was low, indicating that downregulation of Cox4i1 is leptin independent. PPARγ agonist treatment that led to an expected increase in adiponectin and insulin sensitivity was associated with an increase of Cox4i1 expression. In contrast, caloric restriction, which increased adiponectin, but left insulin sensitivity unaffected, did not increase Cox4i1. Cox4i1 expression was already low in STZ mice at 8 weeks when insulin sensitivity was low, but when adiponectin was still normal. These data support the hypothesis that low Cox4i1 is rather associated with impaired insulin signaling than with impaired adipose tissue differentiation alone.
Previously we reported on the association between oxidative stress, obesity and insulin resistance.10, 20 Our current data support a possible mechanistic link between attenuated insulin signaling and oxidative stress in the adipose tissue that may be governed by the decreased expression of the insulin-dependent glucose transporter GLUT4.40 Indeed, we found low Glut4 expression in adipose tissues of leptin-deficient obese mice with diabetes after caloric restriction to be associated with low Cox4i1 despite presence of elevated adiponectin. In turn, an elevation of Glut4 expression in rosiglitazone-treated obese mice increased Cox4i1. This suggests that an improvement of adipose tissue differentiation and glucose uptake is required for restoration of COX complex components, particularly Cox4i1. In STZ mice, a decreased expression of Cox4i1 was already observed at 8 weeks when Glut4 was low, whereas adiponectin expression was still normal. Interestingly, the increase in Pparα in leptin-deficient mice with diabetes after caloric restriction or rosiglitazone treatment correlated with the expression of its target Glut4. Conversely, the decrease in Pparα in STZ mice correlated with the decrease of Glut4. Our combined observations in leptin-deficient obese mice and STZ mice with diabetes showed also a correlation of Cox4i1 with Pparβ/δ, but not with Pparγ, in agreement with earlier observations in Pparβ/δ over-expressing mice.41 Overall, our mouse data link low COX4I1 expression to Pparα/β/δ-dependent GLUT4 expression and insulin signaling. The relation between low PPAR, low GLUT4, high HOMA-IR and low COX4I1 was also observed in white visceral adipose tissues of obese patients. However, as the number of adipose tissue samples was too low to discriminate between obese patients with diabetes and NGTO patients, we studied this relation in monocytes, which are more clinically accessible. Low COX4I1 in monocytes discriminated between obese with diabetes and NGTO patients. Remarkably, considering low IRAK3 in addition to low COX4I1 in our adjusted multivariate analysis model improved prediction of type 2 diabetes in obese patients. This suggests a further synergistic, IRAK3-dependent path to insulin resistance. To this end, we previously demonstrated that IRAK3 is required for the PPAR-dependent antioxidative and anti-inflammatory action of adiponectin.42
Previously a diet high in fat increased the H(2)O(2)-emitting potential of mitochondria, shifted the cellular redox machinery to a more oxidized state, and decreased the redox-buffering capacity in the absence of any change in mitochondrial respiratory function.43 Interestingly, attenuating mitochondrial H(2)O(2) emission completely preserved insulin sensitivity. These findings specifically linked intracellular metabolic balance to the control of insulin sensitivity.44 In addition, reactive oxygen species production was associated with mitochondrial alterations in the muscle of hyperglycemic streptozotocin-treated mice, and normalization of glycemia or antioxidant treatment decreased reactive oxygen species production and restored mitochondrial integrity.45 Thus, our data are in agreement with above literature data showing a close relation between insulin resistance and oxidative stress.
In mouse and human samples, we observed a decrease of several antioxidative systems, such as COX, GPX1 and SOD systems. Interestingly, low Cox4i1 expression was observed when Gpx1 and Sod3 were low, as in DKO mice, or high as in STZ-treated mice, respectively. In aggregate, our data demonstrate that the antioxidant COX complex can be impaired specifically and independently of other antioxidative systems such as Gpx, which detoxifies reactive oxygen species,46 and Sod3, which detoxifies superoxide.47 In humans, COX4I1 was lower in monocytes of obese patients with type 2 diabetes compared with NGTO and lean controls, whereas GPX1 was already lower in NGTO. SOD2 expression was higher in NGTO and patients with diabetes. Overall, our human data confirm that low GPX and high SOD2 in association with obesity and impaired adipogenesis (low adiponectin) do not necessarily lead to an impairment of COX.
As the study was based on cross-sectional data, we cannot make conclusions about possible causal relationship. Second, as we have not gathered data on other interventions than Roux-en-Y bypass, it remains elusive whether other interventions such as sleeve gastrectomy, gastric banding or weight loss induced by pharmacotherapy would yield similar associations and thus provide similar predictive value for long-term consequences of obesity. It has to be said, however, that Roux-en-Y gastric bypass is the preferred and most powerful therapeutic option for obese patients with type 2 diabetes. Although we only followed 17 patients after Roux-en-Y bypass, this small group still allowed appreciating significant beneficial effects of gastric bypass on metabolic parameters, as well as statistically significant changes in gene expressions in monocytes from the same patients, which were comparable to those observed between NGTO and T2D patients.
In conclusion, we found that the expression of COX4I1, an essential element for ATP feedback inhibition of COX, is downregulated in type 2 diabetes. COX4I1 decrease can be independent from the canonical radical detoxification machinery (Gpx1 and Sod3), independent of the IRAK3/adiponectin system and independent of COX biogenesis. COX4I1 decrease is rather linked to decreased glucose uptake and insulin signaling as evidenced by decrease of GLUT4 expression, which may partly depend on PPAR expression. COX4I1 downregulation results in reduced protection against mitochondrial oxidative stress in obesity, and may be related to metabolic dysregulation, ultimately leading to MetS and type 2 diabetes. As this dysregulation was not only found in visceral adipose tissue, but was also detected in circulating monocytes, monitoring COX4I1 expression offers practical tool for patient diagnosis and monitoring. Finally, the COX complex might offer new targets for pharmacotherapy in obesity to prevent or treat MetS and type 2 diabetes.
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and international association for the Study of Obesity. Circulation 2009; 120: 1640–1645.
Grundy SM . Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 2004; 89: 2595–2600.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004; 24: e13–e18.
Muntner P, He J, Chen J, Fonseca V, Whelton PK . Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III). Ann Epidemiol 2004; 14: 686–695.
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH . Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000; 101: 1767–1772.
Ridker PM, Buring JE, Cook NR, Rifai N . C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003; 107: 391–397.
Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2001; 21: 844–848.
Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM et al. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol 2003; 23: 1444–1448.
Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes 2004; 53: 1068–1073.
Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR Jr . Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 2008; 299: 2287–2293.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761.
Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L et al. Adiponectin as a link between type 2 diabetes mellitus and vascular NADPH-oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 2014. e-pub ahead of print 31 December 2014.
Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 2015; 64: 36–48.
Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ . Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005; 111: 932–939.
Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D . Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998; 98: 1487–1494.
Wallace DC . A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005; 39: 359–407.
Poyton RO, McEwen JE . Crosstalk between nuclear and mitochondrial genomes. Annu Rev Biochem 1996; 65: 563–607.
Villani G, Attardi G . In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci USA 1997; 94: 1166–1171.
Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev 2009; 25: 733–739.
Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009; 32: 1302–1307.
Arnold S . Cytochrome c oxidase and its role in neurodegeneration and neuroprotection. Adv Exp Med Biol 2012; 748: 305–339.
Arnold S . The power of life—cytochrome c oxidase takes center stage in metabolic control, cell signalling and survival. Mitochondrion 2012; 12: 46–56.
Williams SL, Valnot I, Rustin P, Taanman JW . Cytochrome c oxidase subassemblies in fibroblast cultures from patients carrying mutations in COX10, SCO1, or SURF1. J Biol Chem 2004; 279: 7462–7469.
Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 2013; 123: 1262–1274.
Cummings DE, Overduin J, Foster-Schubert KE . Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004; 89: 2608–2615.
Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004; 110: 2952–2967.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Hulsmans M, Geeraert B, De Keyzer D, Mertens A, Lannoo M, Vanaudenaerde B et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One 2012; 7: e30414.
Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation 2003; 107: 1640–1646.
Verreth W, De KD, Pelat M, Verhamme P, Ganame J, Bielicki JK et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 2004; 110: 3259–3269.
Ridderstrale M, Carlsson E, Klannemark M, Cederberg A, Kosters C, Tornqvist H et al. FOXC2 mRNA expression and a 5' untranslated region polymorphism of the gene are associated with insulin resistance. Diabetes 2002; 51: 3554–3560.
Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol 1996; 157: 3850–3859.
Salio M, Cerundolo V, Lanzavecchia A . Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol 2000; 30: 705–708.
Cynis H, Kehlen A, Haegele M, Hoffmann T, Heiser U, Fujii M et al. Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. Int J Exp Pathol 2013; 94: 217–225.
Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol 2013; 46: 141–152.
Geeraert B, De Keyzer D, Davey PC, Crombe F, Benhabiles N, Holvoet P . Oxidized low-density lipoprotein-induced expression of ABCA1 in blood monocytes precedes coronary atherosclerosis and is associated with plaque complexity in hypercholesterolemic pigs. J Thromb Haemost 2007; 5: 2529–2536.
Hulsmans M, De Keyzer D, Holvoet P . MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J 2011; 25: 2515–2527.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3, RESEARCH0034.
Wang CH, Wang CC, Huang HC, Wei YH . Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J 2013; 280: 1039–1050.
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM et al. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 2007; 117: 3930–3939.
Hulsmans M, Geeraert B, Arnould T, Tsatsanis C, Holvoet P . PPAR agonist-induced reduction of Mcp1 in atherosclerotic plaques of obese, insulin-resistant mice depends on adiponectin-induced Irak3 expression. PLoS One 2013; 8: e62253.
Rindler PM, Crewe CL, Fernandes J, Kinter M, Szweda LI . Redox regulation of insulin sensitivity due to enhanced fatty acid utilization in the mitochondria. Am J Physiol Heart Circ Physiol 2013; 305: H634–H643.
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009; 119: 573–581.
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008; 118: 789–800.
Kadenbach B, Ramzan R, Vogt S . Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med 2009; 15: 139–147.
Fridovich I . Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 1989; 264: 7761–7764.
Acknowledgements
We thank Roxane Menten for her excellent technical support. This work was funded by the Bijzonder Onderzoeksfonds of the KU Leuven (PF/10/014; Center of Excellence), by the Interdisciplinair Ontwikkelingsfonds—Kennisplatform (KP/12/009) and by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (G0846.11 and Vascular Biology Network). MH is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Van der Schueren, B., Vangoitsenhoven, R., Geeraert, B. et al. Low cytochrome oxidase 4I1 links mitochondrial dysfunction to obesity and type 2 diabetes in humans and mice. Int J Obes 39, 1254–1263 (2015). https://doi.org/10.1038/ijo.2015.58
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2015.58
This article is cited by
-
Identification of key pathways and genes in polycystic ovary syndrome via integrated bioinformatics analysis and prediction of small therapeutic molecules
Reproductive Biology and Endocrinology (2021)
-
Senolytic CAR T cells reverse senescence-associated pathologies
Nature (2020)
-
Targeted Metabolomics Reveals Abnormal Hepatic Energy Metabolism by Depletion of β-Carotene Oxygenase 2 in Mice
Scientific Reports (2017)